the complex choreography of eye development
Florencia Cavodeassi and Stephen Wilson, October 2009
Original paper reference
Dynamic Coupling of Pattern Formation and Morphogenesis in the Developing Vertebrate Retina
If we are to interpret correctly what we see, a precise representation of the visual input received the retina must be formed in our brain. This "visual map" forms during embryogenesis, when retinal neurons (nerve cells) project long processes (axons) to the brain to establish the wiring between the eye and those parts of the brain that process visual information. Each retinal axon targets a very specific area of the superior colliculus (or optic tectum), a region of the brain that interprets visual information, thus generating a map of connections that reproduces the external visual field in the brain. For the visual map to be accurate, the neurons in the retina must be able to interpret where in the retina they are located, and who are their neighbours. We know quite a lot about the genetic "codes" that give the retinal cells their identity but much less about how the retinal cells acquire this knowledge of who they are and where they must go.
Until now, it has been particularly challenging to resolve the mechanisms that instruct positional information in the retina, since this process happens in parallel to the extensive remodelling (or morphogenesis) of tissues that occur during formation of the eye. Using a combination of imaging techniques and genetic manipulations, in this study, we describe how the processes of morphogenesis and allocation of retinal identity are coordinated during maturation of the optic cup.
In a previous study, our collaborators Alexander Picker and Michael Brand proposed that a combination of secreted Fibroblast Growth Factor (Fgf) proteins are required by the eye cells to 'know' where they are. The study determined that retinal cells acquire their identity during a very early stage of eye development, when the nascent eye (optic vesicle/cup) is just beginning to emerge from the brain. This presented a puzzle - Fgfs work through being secreted from one group of cells and received and interpreted by nearby cells - however, during the stage when Fgfs appear to function in the eye, the nascent eye tissues are undergoing complex rearrangements. How then, could this process of morphogenesis be temporally and spatially coordinated with the localised action of Fgfs on retinal cells? This study found that as the prospective eye cells undergo various movements, it brings them transiently into proximity with other tissues that produce Fgfs which can act consequently act upon their temporarily neighbouring nascent retinal cells.
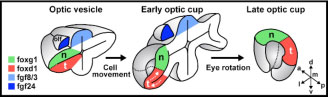
Figure 1: cartoon illustrating the reorganisation of the nasal-temporal axis during maturation of the optic cup. The source of Fgf signals is shown in blue, the future nasal region of the retina is shown in green and the future temporal region in red. The olfactory placode (the future nasal epithelium, dark blue) is one source of Fgfs and the other is the telencephalic region of the forebrain (pale blue).
The retina is divided into two axes - a nasal to temporal axis (nose to ear) and a dorsal to ventral axis (up to down) and Fgf promotes nasal identity. One surprising finding was that Fgfs are produced by tissues dorsal to retina at early stages (Figure 1) suggesting that nasal retina is initially located dorsally in the nascent eye and that the eye somehow rotates by 90 degrees during subsequent stages of development. But how is this shift of the axis effected?
To answer this question required labelling subsets of retinal cells with green fluorescent protein and tracking them in live zebrafish embryos. In this way, it was possible to follow the complex reorganisation movements that transform the initial dorsal-ventral axis of the optic vesicle into the nasal-temporal axis of the mature retina. During this process, the dorsal, future nasal cells of the retina become compacted, the future temporal cells move from ventrally to join the nasal cells in the forming retina and simultaneously the whole optic vesicle rotates to bring the dorsal domain to its final location anteriorly in the eye. Remarkably, this reorganisation is coordinated with a similar shift of the source of Fgfs, which act as choreographers of some aspects of the complex morphogenetic process. In this way, one set of signals (the Fgfs) instructs positional information in the retina and simultaneously ensures that the axis is maintained during the subsequent maturation of the eye.
The study also revealed that many aspects of morphogenesis proceed very well in the absence of Fgfs indicating that other pathways must be involved. We hope that future collaborations will help to identify these additionally choreographers of eye formation.
If you have any further questions, please contact Florencia Cavodeassi or Steve Wilson. Alexander Picker and Michael Brand would also be very happy to receive any questions on the study.
This study was a collaboration with Alexander Picker and Michael Brand and our contribution to the research received financial support from the MRC and Wellcome Trust.