Bianco Lab
understanding the logic of brain circuits that control behaviour
Bianco Lab
understanding the logic of brain circuits that control behaviour
Research
Our aim is to understand fundamental aspects of the structure and operation of the neural circuits that process sensory information to control animal behaviour.
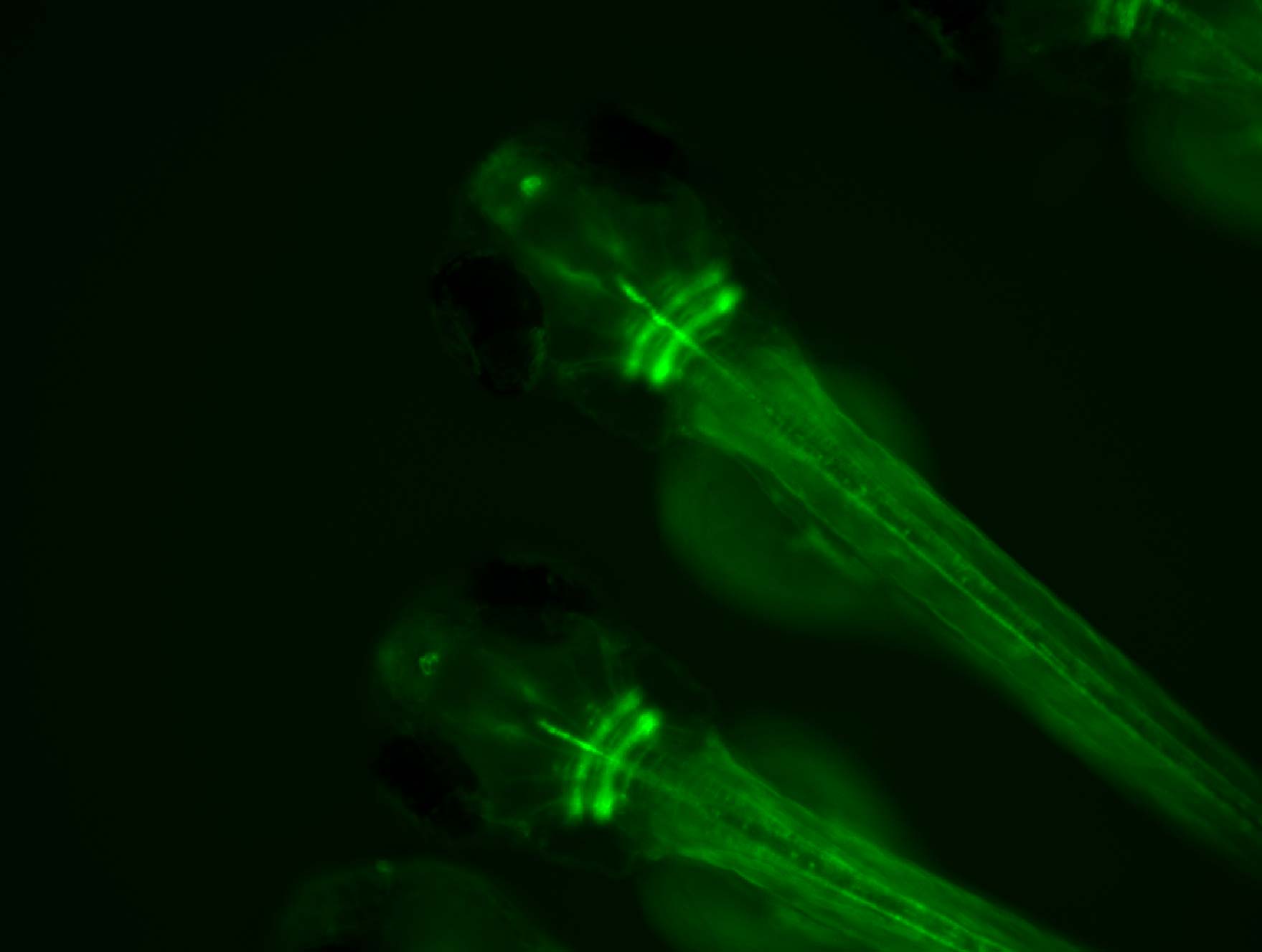
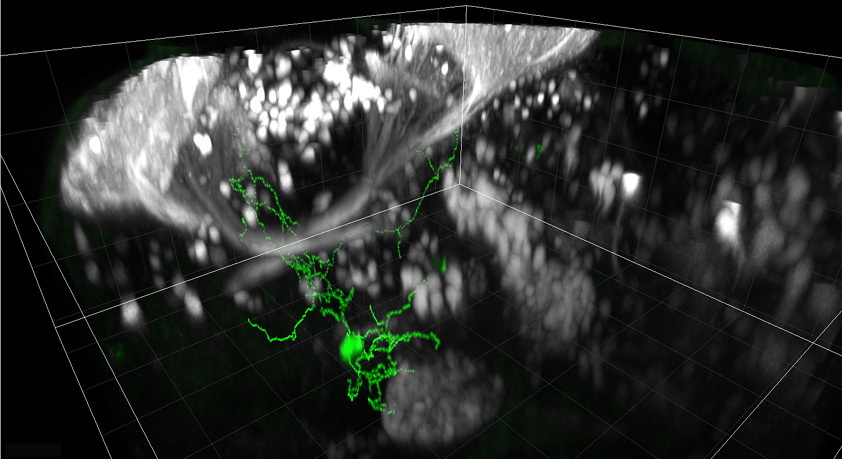
We work with larval zebrafish – a vertebrate model that has a tiny, optically transparent brain – enabling us to use advanced light microscopy techniques to monitor neural activity during behaviour.
2-photon and light sheet microscopes allow us to record activity at single cell resolution throughout the brain of transgenic fish expressing genetically-encoded calcium indicators, which report neural activity with changes in fluorescence.
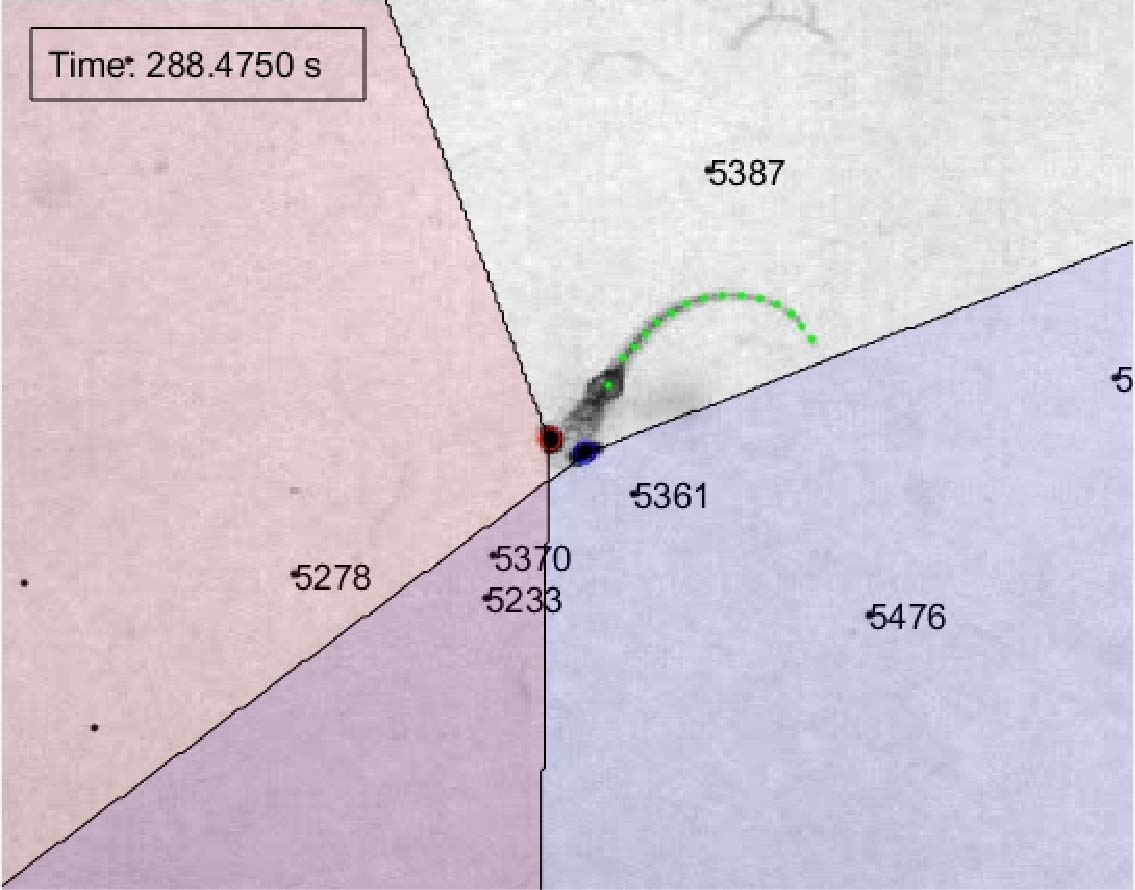
Our focus is on brain circuits that process visual stimuli (for example resembling predators or prey) to control adaptive behavioural responses (such as escape or hunting). We build virtual reality environments for zebrafish and examine neural activity underlying visual perception and the generation of locomotor programmes whilst the animal responds to naturalistic visual stimuli.

News
News
BIANCO LAB NEWS

People
People
PEOPLE
Former members:
Lilie Gailloud, BSc student
Sara Contra, BSc student
Sam Pink, Research Tech
Paride Antinucci, Postdoc
Lukas Rabl, MSci student
Daniel Majd, MSci student
Joanna Lau, PhD student
Jade Serfaty, MSci student
Tom Hagley, MSci student
Alex Bloom, iBSc student
Nicole Maug, MSci student
Pedro Henriques, PhD student
Kristie Leung, iBSc student
Adam Selway, iBSc student
Holly Morley, MSc student
Dammy Onih, MSci student
Charlotte Leigh, iBSc student
Andrien Rajakumar, iBSc student
Yue Wu, MSc student
Niloy Rahmen, iBSc student
Mingxu Shan, MSc student
Sam Jackson, iBSc student

Join us/Contact
Join us/Contact
Join us
We enthusiastically encourage applications from prospective students or postdocs.
Techniques that we specialise in include 2-photon and light sheet microscopy, analysis of population dynamics in neural circuits, design of novel behavioural assays and development of optogenetic approaches for precise control of neural activity in vivo.
Please send an email outlining your research interests, along with your CV, to Isaac.
Phd programmes
Our lab is part of several excellent graduate programmes:
• Wellcome Trust 4yr PhD in Optical Biology
• UCL-Birkbeck MRC Doctoral Training Programme
• London Interdisciplinary Doctoral Programme
• Sainsbury Wellcome Centre PhD Programme
Contact Us
Please contact Isaac Bianco for more information.
For directions to the lab, click here.

Publications
Publications
Publications
Featured PapersSupraspinal commands have a modular organization that is behavioral context specific. Lau JYN, Fitzgerald JE and Bianco IH. Current Biology (2025). 10.1016/j.cub.2025.07.066
We use calcium imaging and statistical modeling to comprehensively survey reticulospinal activity during diverse locomotor behaviors. We find that a small set of functional modules act combinatorially to generate descending locomotor commands. Unexpectedly, this control architecture is stratified according to behavioral context, with three modules being specialised for locomotor control during hunting.
Subsets of extraocular motoneurons produce kinematically distinct saccades during hunting and exploration. Dowell CK, Hawkins T, Bianco IH. Current Biology (2025). 10.1016/j.cub.2024.12.010
We discover that two kinematically distinct types of saccadic eye movement are controlled by specific subsets of extraocular motoneurons. Parallel type-specific and type-agnostic premotor pathways differentially engage motoneuron subsets during visual exploration and hunting.
Kinematically distinct saccades are used in a context-dependent manner by larval zebrafish. Dowell CK, Lau JYN, Antinucci P, Bianco IH. Current Biology (2024). 10.1016/j.cub.2024.08.008
We characterize the repertoire of saccadic eye movements of larval zebrafish and examine their coordination with swims during visuomotor behaviors. Differences in timing, kinematics, and velocity main sequences indicate that different saccade types are controlled by distinct patterns of brainstem activity.
Mirror-assisted light-sheet microscopy: a simple upgrade to enable bi-directional sample excitation. Zylbertal A, Bianco IH. Neurophotonics (2024). doi.org/10.1117/1.NPh.11.3.035006
We describe a simple solution that uses a tiny mirrored prism to illuminate the ~25% of the zebrafish brain that is normally inaccessible to a laterally directed light-sheet.
A recurrent network architecture explains tectal activity dynamics and experience-dependent behaviour. Zylertal A, Bianco IH. eLife (2023). doi.org/10.7554/eLife.78381
We used light-sheet calcium imaging and computational modelling to explore how activity in neural networks affects their internal state and contributes to variability in activity and behaviour.
A calibrated optogenetic toolbox of stable zebrafish opsin lines. Antinucci P*, Dumitrescu AS*, Deleuze C, Morley HJ, Leung K, Hagley T, Kubo F, Baier H, Bianco IH*, Wyart C*. eLife (2020) 9:e54937 10.7554/eLife.54937
We generate nine transgenic lines expressing various optogenetic actuators and calibrate their efficacy using behavioural assays and in vivo electrophysiology.
An interhemispheric neural circuit allowing binocular integration in the optic tectum. Gebhardt C, Auer TO, Henriques PM, Rajan G, Duroure K, Bianco IH*, Del Bene F*. Nature Communications (2019) 10, 5471. doi:10.1038/s41467-019-13484-9
We describe a circuit motif for binocular vision in an animal lacking ipsilateral retinal projections: ‘Inter-tectal neurons’ are required for hunting larvae to strike at prey.
Pretectal neurons control hunting behaviour. Antinucci P, Folgueira M, Bianco IH. eLife (2019) 8 doi.org/10.7554/eLife.48114
We discovered a genetically-accessible population of pretectal neurons that satisfy the criteria of a ‘command system’ controlling hunting routines. These cells are active when zebrafish initiate hunting and optogenetic stimulation of single cells evokes complete hunting sequences in the absence of prey.
Nucleus Isthmi is required to sustain target pursuit during visually guided prey-catching. Henriques PM, Rahman N, Jackson SE, Bianco IH. Current Biology (2019) 29:1771-1786. doi.org/10.1016/j.cub.2019.04.064
We discovered that the nucleus isthmus (NI) is required for zebrafish larvae to sustain (but not initiate) hunting sequences and linked this function to a specific type of NI cell. To our knowledge, this is the first example of a neuron that promotes maintenance of stochastic action sequences.
Bianco IH and Engert F. Visuomotor transformations underlying hunting behavior in zebrafish. Current Biology (2015). 25:1-16. 10.1016/j.cub.2015.01.042
By combining 2-photon calcium imaging with a naturalistic hunting assay, we identified feature-analysing neurons in the optic tectum that selectively respond to optimal conjunctions of prey-like visual features, and bursts of premotor activity in tectal assemblies that precede hunting responses.
All Papers (chronological)Supraspinal commands have a modular organization that is behavioral context specific. Lau JYN, Fitzgerald JE and Bianco IH. Current Biology (2025). 10.1016/j.cub.2025.07.066
Standardized measurements for monitoring and comparing multiphoton microscope systems. Lees RM, Bianco IH, Campbell RAA, Orlova N, Peterka DS, Pichler B, Smith SL, Yatsenko D, Yu CH, Packer AM. Nature Protocols (2025) Nature link
Subsets of extraocular motoneurons produce kinematically distinct saccades during hunting and exploration. Dowell CK, Hawkins T, Bianco IH. Current Biology (2025). 10.1016/j.cub.2024.12.010
Kinematically distinct saccades are used in a context-dependent manner by larval zebrafish. Dowell CK, Lau JYN, Antinucci P, Bianco IH. Current Biology (2024). 10.1016/j.cub.2024.08.008
Mirror-assisted light-sheet microscopy: a simple upgrade to enable bi-directional sample excitation. Zylbertal A, Bianco IH. Neurophotonics (2024). doi.org/10.1117/1.NPh.11.3.035006
A recurrent network architecture explains tectal activity dynamics and experience-dependent behaviour. Zylertal A, Bianco IH. eLife (2023). doi.org/10.7554/eLife.78381
Foxd1 dependent induction of temporal retinal character is required for visual function. Hernández-Bejarano M, Gestri G, Monfries C, Tucker L, Dragomir E, Bianco IH, Bovolenta P, Wilson S, Cavodeassi F. Development (2022). 149(24):dev200938. doi: 10.1242/dev.200938
A Structural Atlas of the Developing Zebrafish Telencephalon Based on Spatially-Restricted Transgene Expression. Turner KJ, Hawkins TA, Henriques PM, Valdivia LE, Bianco IH, Wilson SW, Folgueira M. Front Neuroanat. (2022). 16:840924. doi: 10.3389/fnana.2022.840924
Loss of slc39a14 causes simultaneous manganese hypersensitivity and deficiency in zebrafish. Tuschl K, White RJ, Trivedi C, Valdivia LE, Niklaus S, Bianco IH, Dadswell C, González-Méndez R, Sealy IM, Neuhauss SCF, Houart C, Rihel J, Wilson SW, Busch-Nentwich EM. Dis Model Mech. (2022). 15:dmm044594. doi: 10.1242/dmm.044594.
CNS Hypomyelination Disrupts Axonal Conduction and Behavior in Larval Zebrafish. Madden ME, Suminaite D, Ortiz E, Early JJ, Koudelka S, Livesey MR, Bianco IH, Granato M, Lyons DA. J Neurosci. (2021). 41:9099-9111. doi: 10.1523/JNEUROSCI.0842-21.2021
Myelination induces axonal hotspots of synaptic vesicle fusion that promote sheath growth. Almeida RG, Williamson JM, Madden ME, Early JJ, Voas MG, Talbot WS, Bianco IH, Lyons DA. Curr Biol. (2021) 31:3743-3754.e5. doi: 10.1016/j.cub.2021.06.036
The Rac-GAP alpha2-Chimaerin Signals via CRMP2 and Stathmins in the Development of the Ocular Motor System. Carretero-Rodriguez L, Guðjónsdóttir R, Poparic I, Reilly ML, Chol M, Bianco IH, Chiapello M, Feret R, Deery MJ, Guthrie S. J Neurosci. (2021). 41:6652-6672. doi: 10.1523/JNEUROSCI.0983-19.2021
Real-time 3D movement correction for two-photon imaging in behaving animals. Griffiths VA, Valera AM, Lau JYN, Ros H, Marin B, Baragli C, Coyle D, Evans GJ, Konstantinou G, Younts TJ, Koimtzis T, Srinivas Nadella KMN, Kirkby PA, Bianco IH and Silver RA. Nature Methods (2020). doi.org/10.1038/s41592-020-0851-7
Perspectives in zebrafish research. Edited by: Mione MC, Blader P, Del Bene F, Trompouki E and Bianco IH. Frontiers in Cell and Developmental Biology (2020)
A calibrated optogenetic toolbox of stable zebrafish opsin lines. Antinucci P*, Dumitrescu AS*, Deleuze C, Morley HJ, Leung K, Hagley T, Kubo F, Baier H, Bianco IH*, Wyart C*. eLife (2020) 9:e54937 10.7554/eLife.54937
Anatomy and Connectivity of the Torus Longitudinalis of the Adult Zebrafish. Folgueira M, Riva-Mendoza S, Ferreño-Galmán N, Castro A, Bianco IH, Anadón R and Yáñez J. Front. Neural Circuits (2020) 14:8. doi:10.3389/fncir.2020.00008
An interhemispheric neural circuit allowing binocular integration in the optic tectum. Gebhardt C, Auer TO, Henriques PM, Rajan G, Duroure K, Bianco IH*, Del Bene F*. Nature Communications (2019) 10, 5471. doi:10.1038/s41467-019-13484-9
Pretectal neurons control hunting behaviour. Antinucci P, Folgueira M, Bianco IH. eLife (2019) 8 doi.org/10.7554/eLife.48114
Zebrafish oxytocin neurons drive nocifensive behavior via brainstem premotor targets. Wee CL, Nikitchenko M, Wang WC, Luks-Morgan SJ, Song E, Gagnon JA, Randlett O, Bianco IH, Lacoste AMB, Glushenkova E, Barrios JP, Schier AF, Kunes S, Engert F & Douglass AD. Nature Neuroscience (2019) doi.org/10.1038/s41593-019-0452-x
Nucleus Isthmi is required to sustain target pursuit during visually guided prey-catching. Henriques PM, Rahman N, Jackson SE, Bianco IH. Current Biology (2019) 29:1771-1786. doi.org/10.1016/j.cub.2019.04.064
Cellular-level understanding of supraspinal control: what can be learned from zebrafish? Lau JYN, Bianco IH, Severi KE. Curr Opin Physiol (2019) 8:141 doi.org/10.1016/j.cophys.2019.01.013
Compensatory growth renders Tcf7l1a dispensable for eye formation despite its requirement in eye field specification. Young RM, Hawkins TA, Cavodeassi F, Stickney HL, Schwarz Q, Lawrence LM, Wierzbicki C, Cheng BY, Luo J, Ambrosio EM, Klosner A, Sealy IM, Rowell J, Trivedi CA, Bianco IH, Allende ML, Busch-Nentwich EM, Gestri G, Wilson SW. Elife. (2019) 8. doi: 10.7554/eLife.40093
Gaze-stabilizing central vestibular neurons project asymmetrically to extraocular motoneuron pools. Schoppik D, Bianco IH, Prober DA, Douglass AD, Robson DN, Li JMB, Greenwood JSF, Soucy E, Engert F, Schier AF. J Neurosci. (2017) pii: 1711-17. doi: 10.1523/JNEUROSCI.1711-17.2017
Sensorimotor computation underlying phototaxis in zebrafish. Wolf S, Dubreuil AM, Bertoni T, Böhm UL, Bormuth V, Candelier R, Karpenko S, Hildebrand DGC, Bianco IH, Monasson R, Debrégeas G. Nat Commun. (2017) 8:651. doi: 10.1038/s41467-017-00310-3
Whole-brain serial-section electron microscopy in larval zebrafish. Hildebrand DGC, Cicconet M, Torres RM, Choi W, Quan TM, Moon J, Wetzel AW, Scott Champion A, Graham BJ, Randlett O, Plummer GS, Portugues R, Bianco IH, Saalfeld S, Baden AD, Lillaney K, Burns R, Vogelstein JT, Schier AF, Lee WA, Jeong WK, Lichtman JW, Engert F. Nature (2017). doi:10.1038/nature22356
Targeted Electroporation in Embryonic, Larval, and Adult Zebrafish. Zou M, Friedrich RW, Bianco IH. Methods Mol Biol (2016). 1451:259-69.
Bianco IH and Engert F. Visuomotor transformations underlying hunting behavior in zebrafish. Current Biology (2015). 25:1-16. 10.1016/j.cub.2015.01.042
Tcf7l2-dependent asymmetries in Wnt signalling mediate the left-right asymmetric differentiation of habenular neurons. Hüsken U, Stickney HL, Roussigne M, Beretta CA, Brinkmann I, Young RM, Bianco IH, Tsalavouta M, Zigman M, Hawkins TA, Wen L, Zhang B, Blader P, Lin S, Wilson SW, Carl M. Current Biology (2014). 24:2217-27.
Encoding asymmetry within neural circuits. Concha MC, Bianco IH, Wilson SW. Nature Reviews Neuroscience. (2012). 13:832-43.
The tangential nucleus controls a gravito-inertial vestibulo-ocular reflex. Bianco IH, Ma L-H, Schoppik D, Robson DN, Orger MB, Beck JC, Li JM, Schier AF, Engert F, Baker R. Current Biology (2012). 22:1285-95.
Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Bianco IH, Kampff AR, Engert F. Front. Syst. Neurosci. (2011). 5:101.
Focal electroporation in zebrafish embryos and larvae. Tawk M, Bianco IH, Clarke JD. Methods Mol Biol. (2009). 546:145-51.
Nodal signalling imposes left-right asymmetry upon neurogenesis in the habenular nuclei. Roussigné M, Bianco IH, Wilson SW, Blader P. Development. (2009). 136:1549-57.
The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Bianco IH, Wilson SW. Philos Trans R Soc Lond B Biol Sci. (2009). 364:1005-20.
Brain asymmetry is encoded at the level of axon terminal morphology. Bianco IH, Carl M, Russell C, Clarke JD, Wilson SW. Neural Dev. (2008). 3:9.
Wnt/Axin1/beta-catenin signaling regulates asymmetric nodal activation, elaboration, and concordance of CNS asymmetries. Carl M, Bianco IH, Bajoghli B, Aghaallaei N, Czerny T, Wilson SW. Neuron. (2007). 55:393-405.
fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Barth KA, Miklosi A, Watkins J, Bianco IH, Wilson SW, Andrew RJ. Current Biology. (2005). 15:844-50.
Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Aizawa H, Bianco IH, Hamaoka T, Miyashita T, Uemura O, Concha ML, Russell C, Wilson SW, Okamoto H. Current Biology. (2005). 15:238-43.
Kinetics of a cellular nitric oxide/cGMP/phosphodiesterase-5 pathway. Mo E, Amin H, Bianco IH, Garthwaite J. J Biol Chem. (2004). 279:26149-58.