headloop pcr- a simple sequencing-free tool to validate guide RNAs
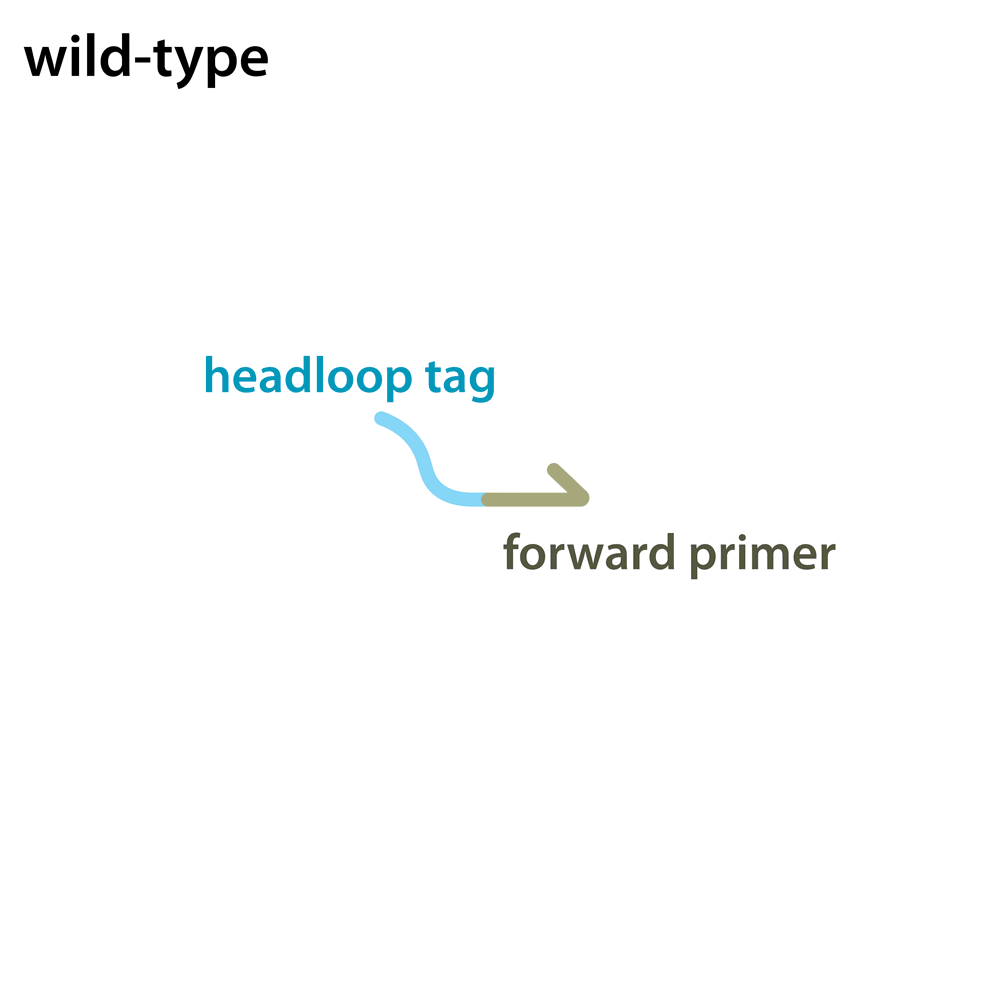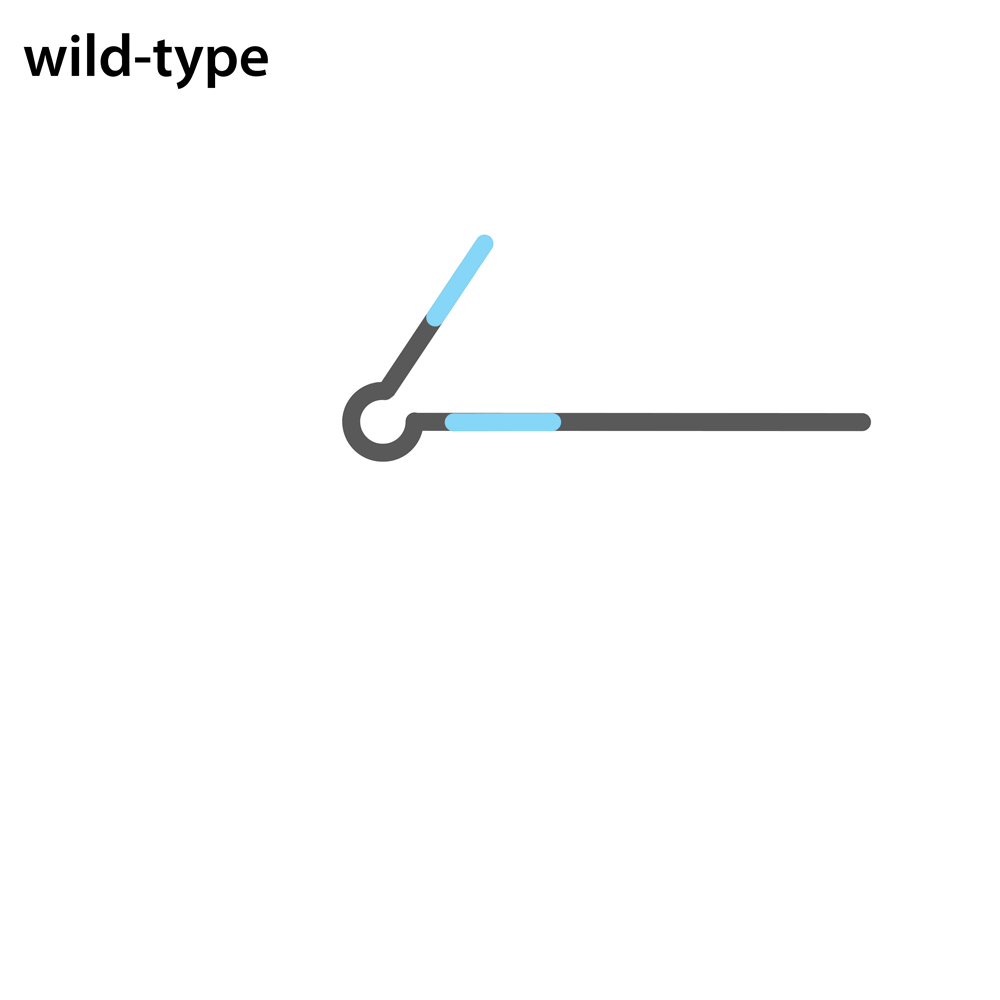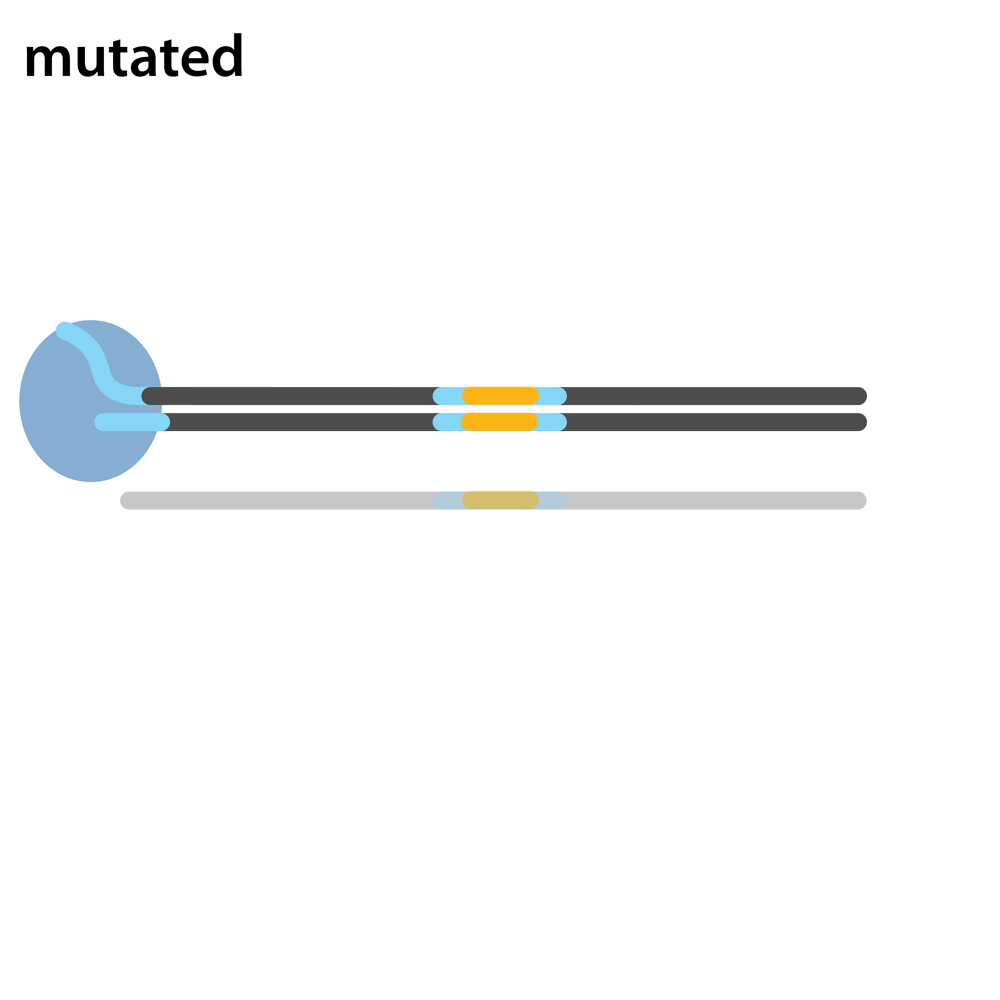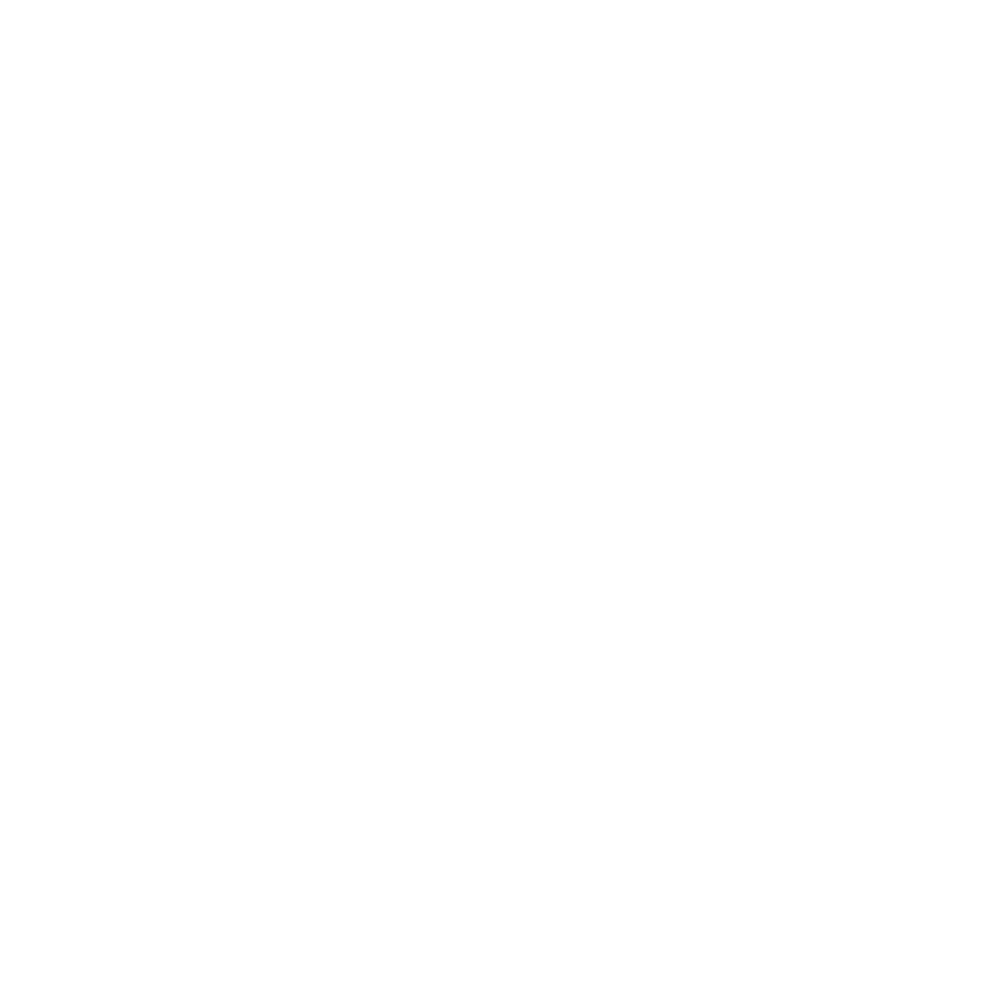
Headloop PCR works by suppressing amplification of a known wildtype haplotype (see GIF) using a target sequence-specific tagged primer to form a stable hairpin. This doesn’t affect mutant haplotypes, rendering the PCR allele-specific without a priori knowledge of the lesion. This can be used to quantify the efficiency of CRISPR/Cas9 RNPs, and to identify and sequence stably inherited alleles in F1 embryos.
The MethoD
A headloop tag sequence that is complementary to the target is added to a PCR primer flanking the target site. During PCR, after the second strand is made, that tag is able to bind to the target sequence within the same molecule and prime extension by the polymerase. This generates a stable hairpin product. If the target sequence is mutated, the headloop tag cannot bind, and the strand is available for further amplification. In samples where there is a mixture of wildtype and mutant alleles, headloop PCR suppresses amplication of wildtype haplotypes but doesn’t affect mutant haplotypes. The amount of amplification correlates well with the proportion of mutant haplotypes in a sample. If the mixed sample comes from an F0 outcross (i.e. is heterozygous for an unknown mutant allele), it can be sequenced to determine the lesion.
protocol
A detailed protocol for headloop PCR is available at Bio-protocol (https://bio-protocol.org/prep930)
A Python-based tool for designing headloop tags is available at Github (https://github.com/GTPowell21/Headloop).
For further details about headloop PCR and its use in testing CRISPR/Cas9 guides please see our article at eLife: Kroll et al. 2021 (https://doi.org/10.7554/eLife.59683).
happy headlooping!