
2hpf
by Afnan M Abdullahi (Attwell Lab)
This image of 2hpf embryos was taken by an In2Science student on the "hubble" scope. Afnan won the "under the lens" category of the Abcam photo competition with this ghostly pic.

Calretinin 3dpf
By Monica Folgueira
A lateral view of a 3dpf zebrafish larvae labelled with anti-Calretinin(green) to label a subset of neurons in the brain and anti-tubulin antibodies(red) to label axonal tracts.

GFP positive tectal neurons with axons(red) and neuropil(white)
By Monica Folgueira
A dorsal view of a 4dpf Tg(1.4dlx5a-6a:GFP) transgenic larvae labelled with anti-GFP(green), anti-tubulin(red) to label axons and anti-SV2(white) to label neuropil. This transgeic labels neurons throughout the telencephalon, optic tectum and cerebellum.

Axons and neuropil (dorsal I)
By Kate Turner
Dorsal view of an un-dissected 4dpf embryo labelled with anti-tubulin antibody(green) to show axons and anti-SV2(magenta) to label neuropil.

Tg(1.4dlx5a-6a:GFP) embryo stained against GFP, SV2 and acetylated tubulin
By Monica Folgueira
A lateral view of a 4dpf Tg(1.4dlx5a-6a:GFP) transgenic larvae labelled with anti-GFP(green), anti-tubulin(red) to label axons and anti-SV2(white) to label neuropil. This transgeic labels neurons throughout the telencephalon, optic tectum and cerebellum.

Neuronal tract labelling (green) and neuropil (red) in 4dpf brain, lateral view
By Jay Patel
A lateral view of a 4dpf zebrafish larvae labelled with anti-SV2(red) to label neuropil and anti-tubulin(green) to label axonal tracts.

Axons and Neuropil (Dorsal II)
By Kate Turner
Dorsal view of an dissected 4dpf embryo labelled with anti-tubulin antibody(green) to show axons and anti-SV2(magenta) to label neuropil.

Axons and Neuropil (Lateral I)
By Kate Turner
Lateralview of an un-dissected 4dpf embryo labelled with anti-tubulin antibody(green) to show axons and anti-SV2(magenta) to label neuropil.

Tg(vmat2:GFP)
By Kate Turner
Dorsal view of a 5dpf Tg(vmat2:GFP) larvae. GFP expression in pineal organ, telencephalon, olfactory bulbs, hindbrain and processes in the tectum, telencephalon and cerebellum (green).

Glycinergic neurons in the spinal cord
By Kate Turner
Lateral view of a 24hpf Tg(gly2:GFP) zebrafish embryo with glycinergic neurons labelled in green and axons labelled with anti-tubulin(red).
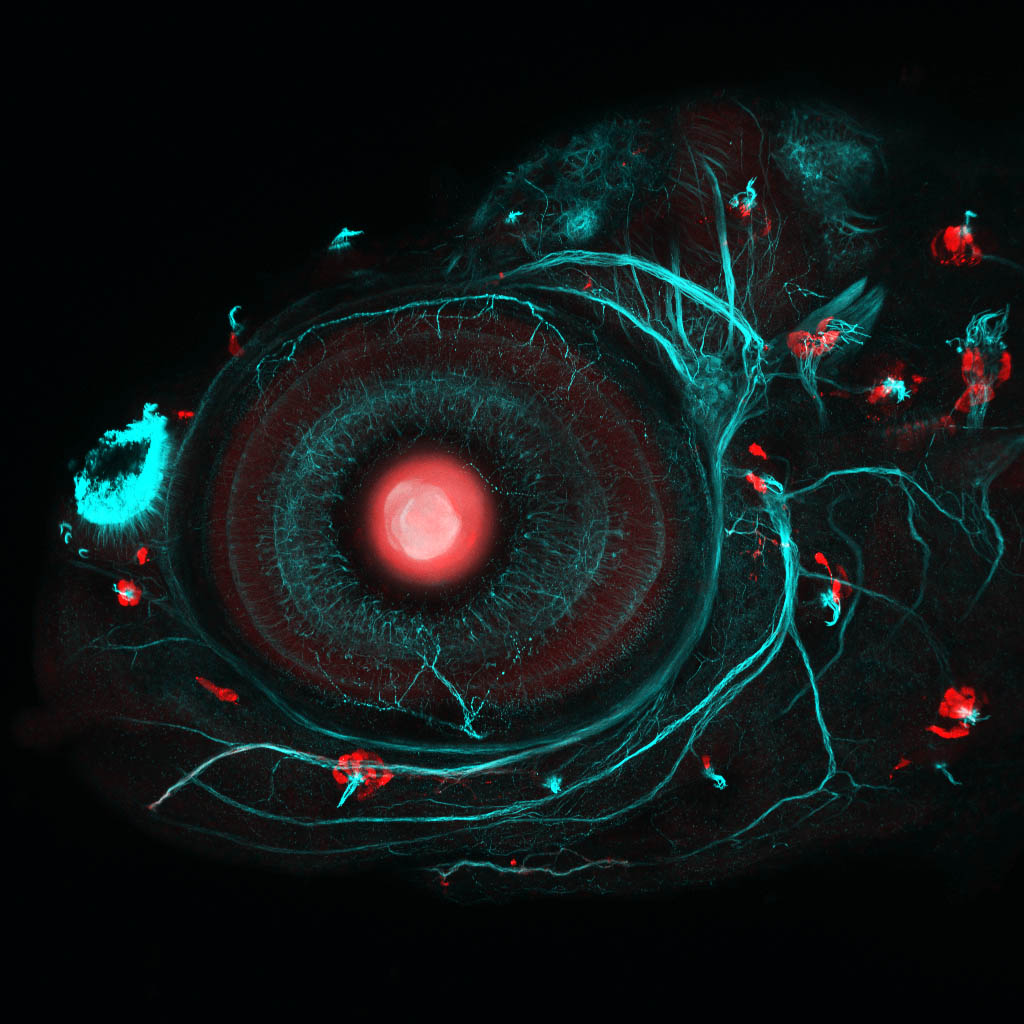
Little moon
By Ingrid Lekk
The pineal complex of the larval zebrafish. In a four day-old zebrafish the photoreceptive pineal complex is located at the dorsal surface of the brain. During development the medially positioned pineal organ gives rise to a left-sided parapineal that sends axon projections to adjacent diencephalic regions and regulates the development of left-right asymmetries there.

Glycinergic neurons in the spinal cord(48hpf)
By Kate Turner
Lateral view of a 48hpf Tg(gly2:GFP) zebrafish embryo with glycinergic neurons labelled in green and axons labelled with anti-tubulin(red).

Bodipy labelled somites and notocord
by Arantza Barrios
Lateral view of the trunk of a zebrafish larvae labelled with the fluorescent dye, Bodipy(green). This dye labels the membranes of the notocord and somite cells and reveals the characteristic "coin" shape of the notocord cells.

Dextran and Bodipy labelled cells in the mesoderm of a living fish embryo
By Arantza Barrios

Dorsal view of the forebrain of a WT zebrafish embryo
By Ana Faro
This figure is a monochrome composite of a confocal micrograph showing the dorsal view of the forebrain of a wild-type zebrafish embryo at 4 days post-fertilization. Wild-type specimens display noticeable neuroanatomical asymmetries of the habenular nuclei in the forebrain, which can be visualised with the help of antibodies labelling different structures. Here the arrangement of the axons, synaptic neuropil and a subtype of habenular neurons are predominantly enriched in the left side of the brain.

Dorsal view of the forebrain of a asymmetry mutant zebrafish
By Ana Faro
This figure is a monochrome composite of a confocal micrograph showing the dorsal view of the forebrain of a mutant zebrafish embryo at 4 days post-fertilization. unlike the wild-type specimens that display noticeable neuroanatomical asymmetries of the habenular nuclei in the forebrain, this mutant larvae exhibits symmetrical habenulae. The axons, synaptic neuropil and a subtype of habenular neurons are the same on the left and right side of the brain.

Habenula nucleus of the zebrafish
By Ana Faro and Tom Hawkins
This image shows the habenular nucleus of a zebrafish embryo 4 days post-fertilization. The habenular nucleus is a part of the epithalamus which connects the limbic system to other regions of the brain.
The arrangement of the habenular axons is highlighted in magenta in this image, and the synaptic neuropil in cyan. Neurons expressing the gene left-over (lov) are highlighted in green, and are predominantly enriched in the left habenula. Different neuronal subtypes, with a typical molecular signature and neural connectivity pattern, are produced in different ratios in the left and right habenular nucleus and confer their unique asymmetric nature.

Habenular nucleus of the zebrafish , composition.
By Ana Faro
This figure is a composition of two intercalated confocal micrographs showing the detail view of the habenular nucleus of a wild-type and a mutant zebrafish embryo. The habenular nucleus is a part of the epithalamus which connects the limbic system (responsible for instinct and mood) to other regions of the brain. Wild-type specimens display noticeable neuroanatomical asymmetries of the habenular nuclei, which can be visualised with the help of fluorescent antibodies labelling different structures in the brain. The arrangement of the habenular axons is shown in magenta and the synaptic neuropil is highlighted in cyan. A subtype of habenular neurons, predominantly enriched in the left side of the brain, express a unique molecular marker and are shown here as green.

Habenular nucleus, zebrafish.
By Ana Faro
This figure shows an abnormally symmetric habenular nucleus of a mutant zebrafish embryo 4 days post-fertilization. The habenular nucleus is a part of the epithalamus which connects the limbic system (responsible for instinct and mood) to other regions of the brain. The arrangement of the habenular axons is highlighted in cyan and a subtype of neurons normally enriched in the left epithalamus is highlighted in magenta. In wild-type embryos, different neuronal subtypes, with a typical molecular signature and neural connectivity pattern, are produced in different ratios in the left and right habenular nucleus thus conferring a unique asymmetric nature. Nevertheless, there are naturally occurring mutants where this pattern of asymmetry is disrupted. These mutants are used as invaluable tools to understand the molecular mechanisms governing brain lateralization during embryonic development.

Forebrain asymmetry in zebrafish
By Ana Faro
This image shows a dorsal view of the forebrain of a 4 days post-fertilization zebrafish larvae labelled for Synaptic Vesicle Protein 2 (SV2). SV2 proteins are membrane glycoproteins that mediate synaptic transmission by regulating cytoplasmic calcium levels in nerve terminals during repetitive stimulation. Therefore, they can be used to identify synaptic boutons (pre-synaptic terminals) and help us visualise the synaptic networks in the brain. Wild-type specimens display noticeable asymmetries of their synaptic network.

Epithalamus of the developing zebrafish
By Ana Faro
This image shows the epithalamus of a zebrafish larvae 4 days post-fertilization. Two regions of the epithalamus (which connects the limbic system, responsible for instinct and mood, to other regions of the brain) are shown: axons of the habenular are in purple and the parapineal organ in yellow. The parapineal is an accessory organ to the pineal gland and is thought to have a rudimentary light sensitive function. It represents the most overt asymmetric feature found in any vertebrate brain, as it is only present in the left side of the epithalamus and sends axonal projections exclusively to the left habenula.

AF4
by Kate Turner
Confocal micrograph of a double transgenic larva showing axons in the optic tract in magenta and neurons in the prethalamus in green forming one of the arborisation fields of the retino-fugal pathway.

Brain Lymphatic Endothelial Cells along blood vasculature
by Shannon Shibata-Germanos & Tom Hawkins
Brightly coloured Brain Lymphatic Endothelial Cells along blood vasculature (magenta) on the surface of the zebrafish brain’s Optic Tectum.

Neurons and axons in the hindbrain
by David Lyons (Clarke Lab

Eye field
By Florencia Cavodeassi
Dorsal view of the anterior neural plate, immunostained to reveal the eye field, the primordium of the eyes (red, Tg{rx3:GFP}) and the distribution of filamentus actin (green, phalloidin)

Evagination of the optic vesicles
By Florencia Cavodeassi
Frontal view of the anterior neural plate at the onset of evagination of the optic vesicles (red, Tg{rx3:GFP}; green, F-actin).
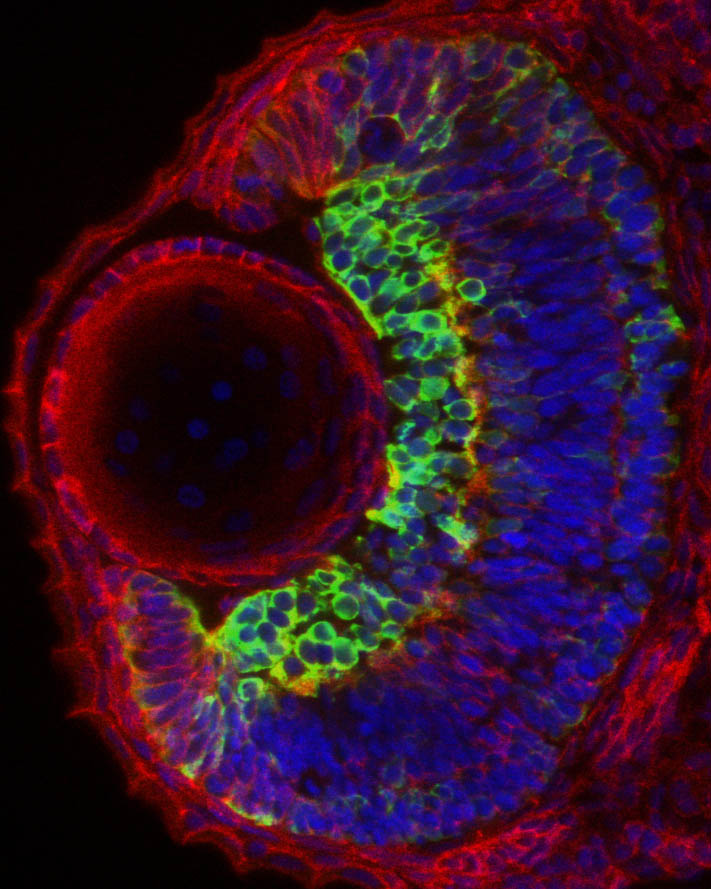
Eye architecture
By Florencia Cavodeassi
Section through and eye from a 2 days old embryo, immunostained to show the general architecture of the eye (red, ß-catenin), the nuclei of all the cells (blue) and a subset of the differentiating neurons in the retina (green, labelled by the expression of the transgenic line Tg{ath5:GFP}.

Frontal views of wildtype, Cyclops and one-eye-pinhead embryos
By Ichiro Masai

Twin zebrafish
By Ingrid Lekk
30hpf WT embryo.
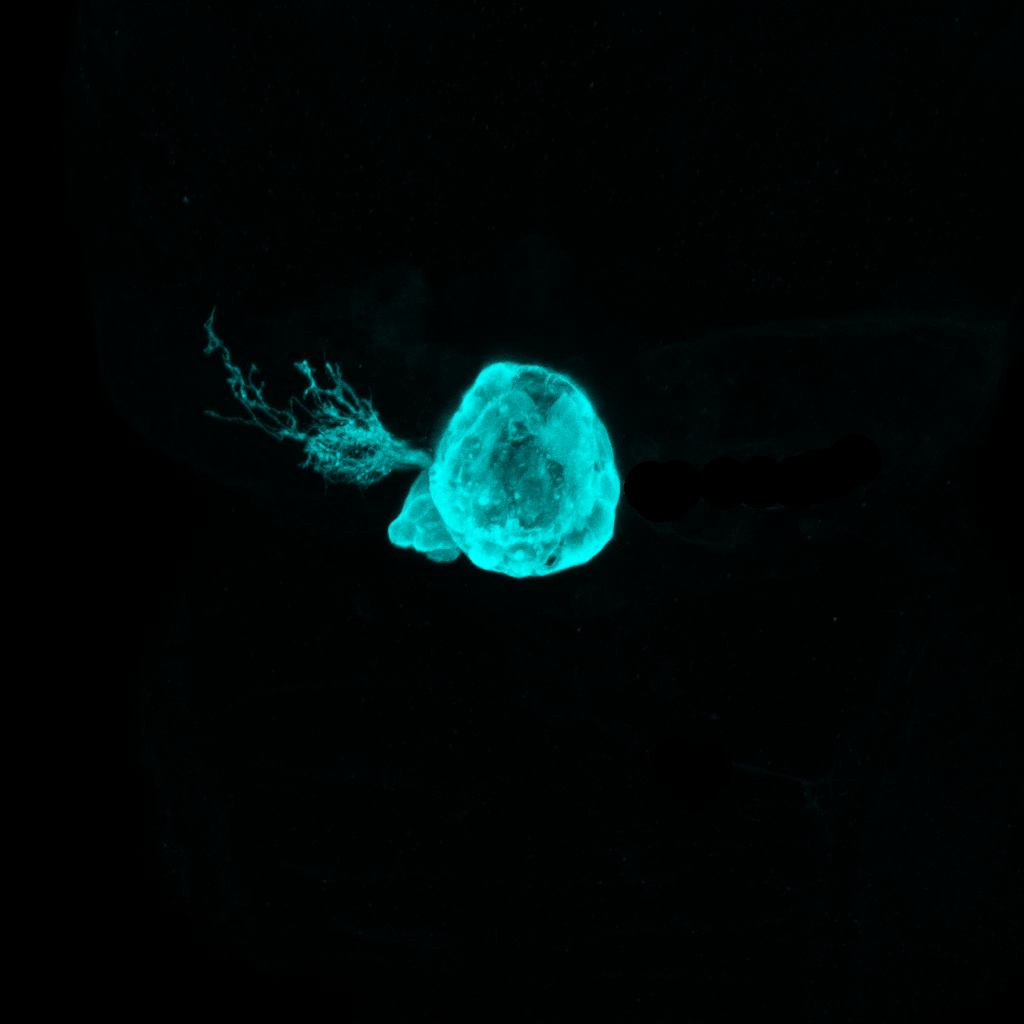
Zebrafish dorsal diencephalon at 4dpf
By Ingrid Lekk
Tg(FoxD3:GFP;flh:GFP), immunolabelling for GFP (grey), acetylated tubulin (magenta) and SV2 (cyan).
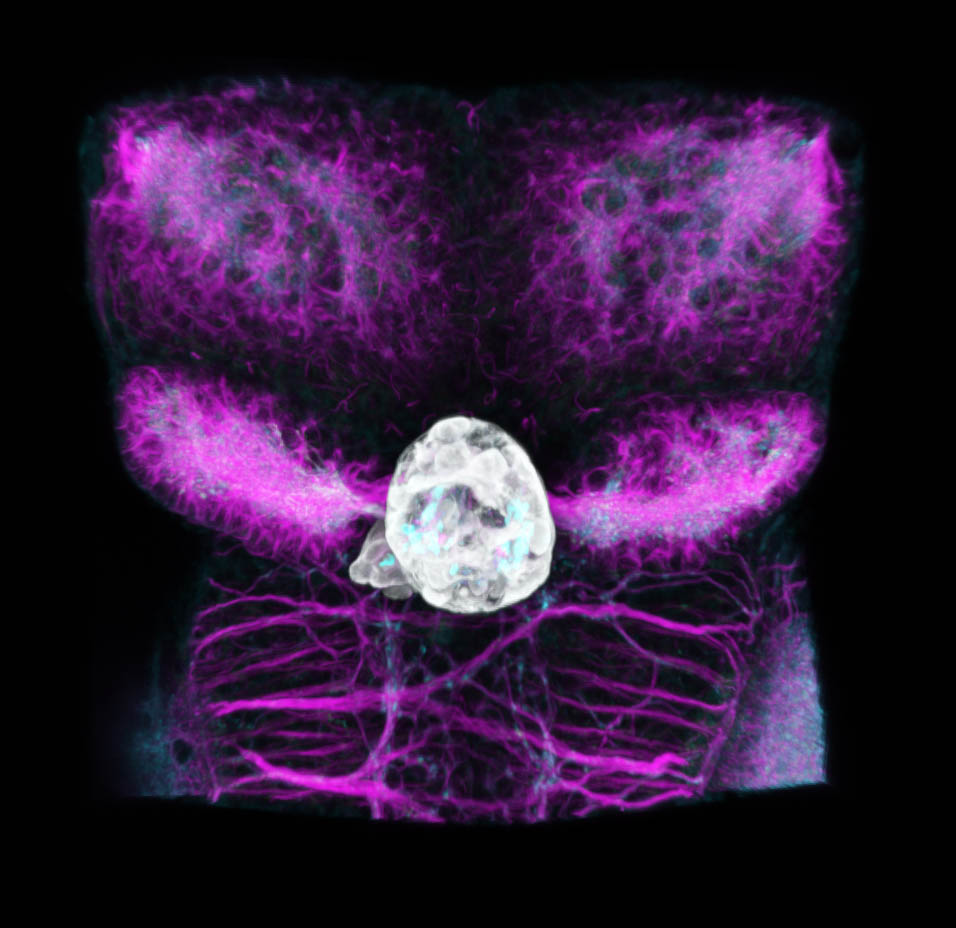
Zebrafish eye and neuromasts
By Ingrid Lekk
The unknown Gal4 transgenic crossed with Tg(UAS:RFP).
A lateral view of a 4dpf transgenic Gal4 zebrafish larvae made using Crispr/Cas labelled with anti-RFP(red) and anti-tubulin(cyan).
This beautiful image won a Wellcome Image Award.
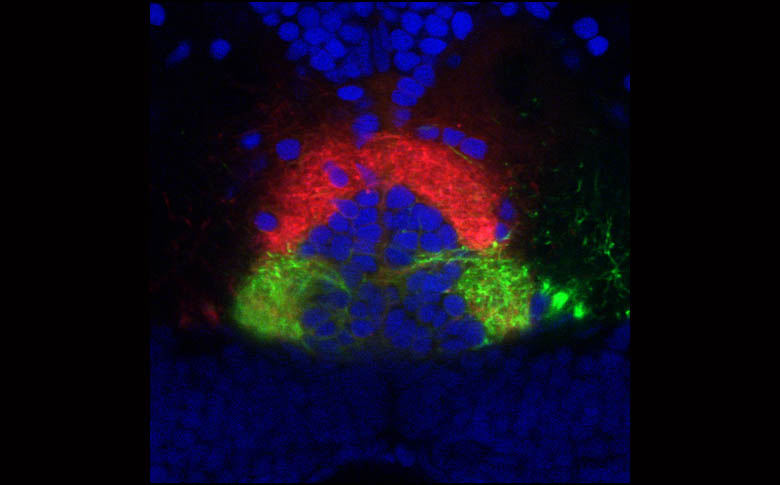
Innervation of the Interpeduncular nucleus.
By Isaac Bianco
Innervation of the IPN by habenula axons labelled with DiI and DiO. Cell nuclei labelled with DAPI.

Optic nerve zebrafish
By Kate Turner
Retinal ganglion cell axons cross at the optic chiasm as they make their way to the to the optic tectum. Confocal micrograph of the brain of a transgenic 60 hour old zebrafish embryo viewed from a ventral aspect. Some neurons express the green fluorescent protein (GFP) - (green) under the control of a specific promotor, which drives expression in retinal ganglion cells, neuromasts, habenulae and the optic tectum. Tracts and axons have been labeled using antibodies against tubulin (red).

Zebrafish sensory neuromasts
By Kate Turner
Sensory neuromast cells of the zebrafish lateral line and ear detect vibrations in the water.
Lateral view of a Tg(Brn3a:GFP) 4dpf larvae. In addition to the neuromasts (green spots around periphery of fish), neurons in the retina, habenula and optic tectum also express green fluorescent protein (GFP).
Neuronal tracts and axons have been labelled using anti-tubulin (red). Tiny cilia (hair-like structures) also labelled with tubulin can be seen supported by the sensory neuromast cells.

Asymmetric habenula afferents
By Kate Turner
Dorsal view of a 4dpf tg(cldnb:lynGFP) larvae labelled with anti-GFP(green) and anti-SV2(magenta). This confocal micrograph shows afferent axons from the forebrain innervating the left dorsal habenula nucleus.

Single axonal process
By Kate Turner
Single axonal process in the zebrafish hindbrain labelled with anti-RFP. This neuron was labelled with a mosaic labelling technique that uses the Crispr/Cas system to convert GFP transgenic lines to Gal4.

Dopaminergic neurons
By Kate Turner
Lateral view of a 4dpfTg(slc6a3:GFP) larvae labelled with anti-GFP(green), anti-tubulin(cyan) and anti-SV2(magenta).

GABAergic and Glutamatergic neurons in the zebrafish brain
By Kate Turner
A 4 day old zebrafish larvae viewed from a ventral aspect (below). Both inhibitory and excitatory neurons can be seen. Inhibitory neurons (green) use gamma-Aminobutyric acid (GABA) and excitatory neurons (magenta) use glutamate as transmitters.
This orientation shows clearly the GABAergic neurons in the subpallium and the hypothalamus.

GABAergic and Glutamatergic neurons (Lateral view)
By Kate Turner
The forebrain of a 4 day old zebrafish larvae viewed from the left hand side. Both inhibitory and excitatory neurons can be seen. Inhibitory neurons (green) use gamma-Aminobutyric acid (GABA) and excitatory neurons (magenta) use glutamate as transmitters. The extensive neural network has been labelled with an antibody for tubulin, a cytoskeletal protein (cyan).

GABAergic neurons in the zebrafish
By Kate Turner
Inhibitory neurons in the zebrafish forebrain using gamma-Aminobutyric acid (GABA) as a neurotransmitter.
Confocal Micrograph of a 4 day old transgenic zebrafish viewed from a ventral aspect (anterior to the top). This transgenic larvae expresses green fluorescent protein (GFP) in a subset of GABAergic neurons (green). Axonal tracts are shown in cyan. This image shows many GABAergic neurons expressing GFP throughout the ventral forebrain including in the sub-pallium, pre-optic areas and hypothalamic lobes. In order to see the neuroanatomy better the skin, eyes and jaw of the larvae have been removed post-fixation.
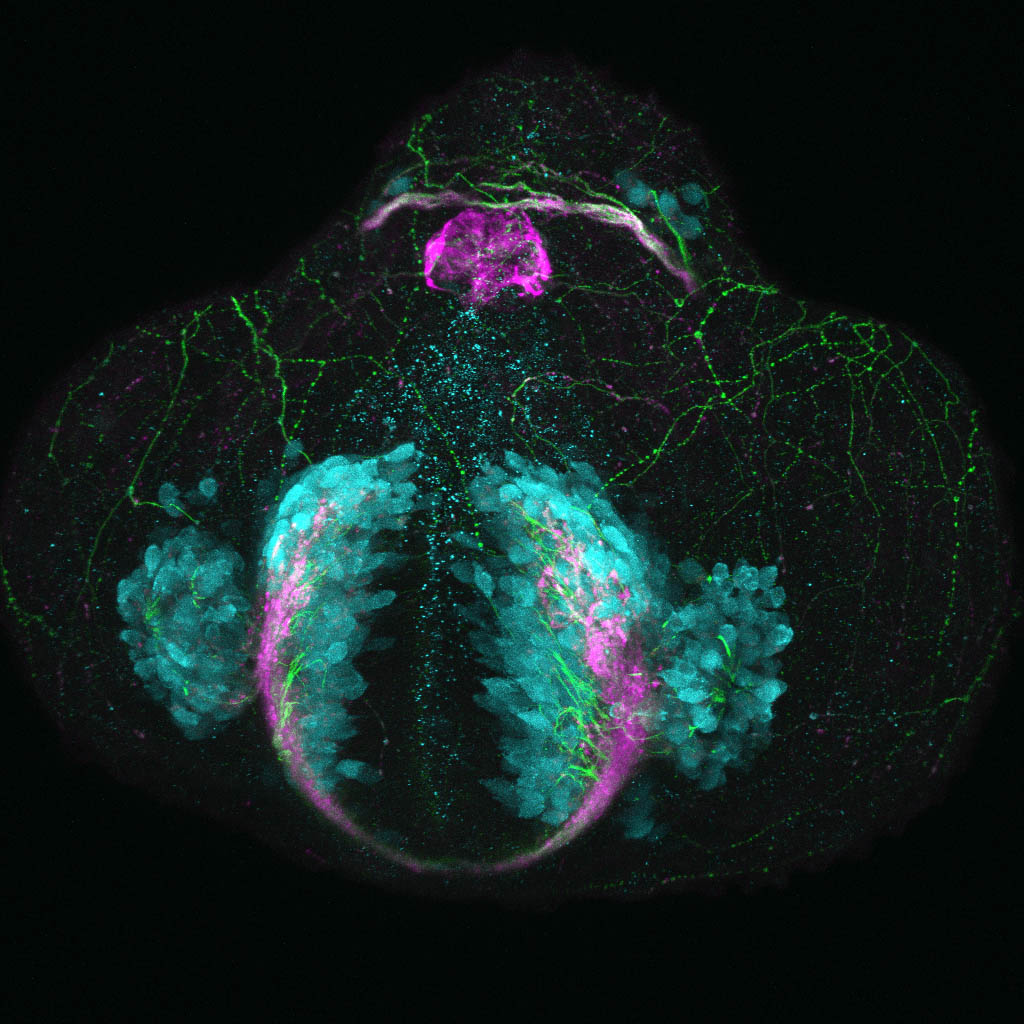
Glutamatergic neurons in the zebrafish telencephalon
By Kate Turner
Neurons in the telencephalon of a 24 hour old zebrafish embryo starting to express a glutamate receptor gene labelled in the Tg(vglut2a:DSRed) transgenic line.
Confocal micrograph of a zebrafish embryo 24 hours post fertilisation viewed from a frontal aspect. Glutamatergic neurons are shown in cyan, neuropil in magenta, and axonal tracts in green. Even at this very early stage of development neurons in the olfactory epithelium, developing telencephalon and dorsal diencephalon are already expressing glutamate receptor vglut2a.

Glycinergic neurons in the zebrafish spinal cord
By Kate Turner
Glycinergic neurons (a subtype of neurons which use glycine as a signalling protein) in a 48 hour old zebrafish embryo, viewed from a lateral aspect. Glycine is a major inhibitory neurotransmitter in the spinal cord and hindbrain. This embryo is labelled to show glycinergic neurons in green and axonal tracts in red. The first glycinergic neurons in the zebrafish spinal cord start to differentiate cordally and a wave of differentiation spreads rostrally (near the front end of the body) between 24 and 48 hours post fertilisation. By 48 hours post fertilisation glycinergic neurons are evident throughout the spinal cord and hindbrain.

Axonal tracts and neuopil(Lateral II)
By Kate Turner
Confocal micrograph of a 4 day old zebrafish larvae labelled with tubulin(green) and anti-SV2 (magenta) antibodies to label axonal tracts and neuropil (clusters of axons) respectively. The larval brain is already well developed with most major tracts established at this stage of development.

Glutamatergic neurons in the preoptic area
By Kate Turner
The forebrain of a 3 day old Tg(lhx5:GFP) zebrafish larvae viewed from Ventral. Excitatory neurons (magenta) that use glutamate as a neurotransmitter are labelled using Tg(vglut2a:DSred). The extensive neural network has been labelled with an antibody for tubulin, a cytoskeletal protein (cyan). Overlap of GFP with vglut2a in the preoptic area shows that many of these cells are glutamatergic.

lhx5:GFP/vglut2a 48hpf dorsal view
By Kate Turner
The forebrain of a 48 hour old Tg(lhx5:GFP) zebrafish larvae viewed from dorsal. Excitatory neurons (magenta) that use glutamate as a neurotransmitter are labelled using Tg(vglut2a:DSred). The extensive neural network has been labelled with an antibody for tubulin, a cytoskeletal protein (cyan). The habenula nuclei label with vglut2a and GFP+ve afferent axons can be seen growing into the epithalamus.

lhx5:GFP/vglut2a 48hpf lateral view
The forebrain of a 48 hour old Tg(lhx5:GFP) zebrafish larvae viewed from lateral. Excitatory neurons (magenta) that use glutamate as a neurotransmitter are labelled using Tg(vglut2a:DSred). The extensive neural network has been labelled with an antibody for tubulin, a cytoskeletal protein (cyan). Overlap of GFP with vglut2a in the preoptic area shows that many of these cells are glutamatergic.

Zebrafish mechanosensory tail cell
By Kate Turner
A mechanosensory neuron with dendrites projecting broadly to cover the entire surface of the tail fin.
This image is a confocal micrograph of a mechanosensory neuron from a 6 day old zebrafish larvae tail. This image is a composite of 5 adjacent high resolution confocal stacks to try and show the entire dendritic morphology of this neuron.

Optic tract
By Kate Turner
The retinofugal pathway or optic tract labelled in a 6dpf Tg(atoh7:RFP) transgenic larvae. Labelled with anti-RFP antibody.

Visual afferents
By Kate Turner
The retinofugal pathway or optic tract labelled in a 6dpf Tg(cldnb:lynGFP):Tg(atoh7:RFP) transgenic larvae. Labelled with anti-GFP and anti-RFP antibody.

Mechanosensory head cell
By Kate Turner
Single mechanosensory cell and its extensive processes in the head of a zebrafish labelled with anti-RFP. This neuron was labelled with a mosaic labelling technique that uses the Crispr/Cas system to convert GFP transgenic lines to Gal4.
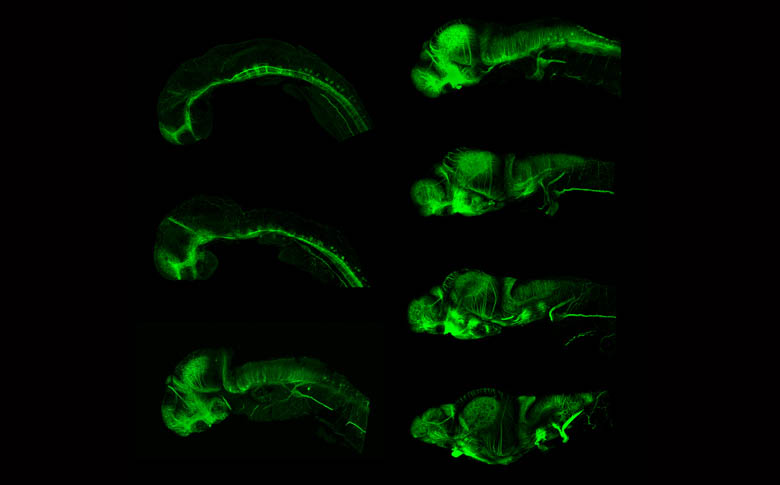
Development of the axonal scaffold
By Kate Turner
Time series showing the development of the axon scaffold of the zebrafish brain.

Axons in a 48hpf embryo
By Kate Turner
Lateral view of a 48 hour old zebrafish embryo stained with acetylated tubulin antibody that labels all of the axonal pathways in the embryo.

Tg(vglut2a:DsRed) 4dpf ventral
By Kate Turner
Ventral view of a 4dpf Tg(vglut2a:DsRed) larvae labelled with anti-DsRed(cyan), anti-SV2(magenta) and anti-tubulin(green). This transgenic line labels neurons that utilise glutamate as a neurotransmitter.

Glutamatergic cells in the early embryo
Lateral view of a 48hpf Tg(vglut2a:DSRed) embryo labelled with anti-DSRed(cyan), anti-SV2(magenta) and anti-tubulin(green) antibodies. This transgenic line labels neurons that use glutamate as a neurotransmitter.

Glutamatergic neurons in the zebrafish forebrain
Glutamatergic neurons in the dorsal forebrain. Confocal micrograph of a 4 day old transgenic zebrafish viewed from a dorsal aspect. Glutamatergic neurons are shown in cyan, neuropil in magenta, and axonal tracts are labelled green. Neurons in the paired habenula nuclei in the dorsal diencephalon are strongly glutamatergic along with neurons in the olfactory bulbs, telencephalon and pretectum.

Glutamatergic neurons in the early zebrafish forebrain
Glutamatergic neurons in the dorsal forebrain. Confocal micrograph of a 48 hour old transgenic zebrafish viewed from a dorsal aspect. Glutamatergic neurons are shown in cyan, neuropil in magenta, and axonal tracts are labelled green. Neurons in the paired habenula nuclei in the dorsal diencephalon are strongly glutamatergic along with neurons in the olfactory bulbs, telencephalon and pretectum.
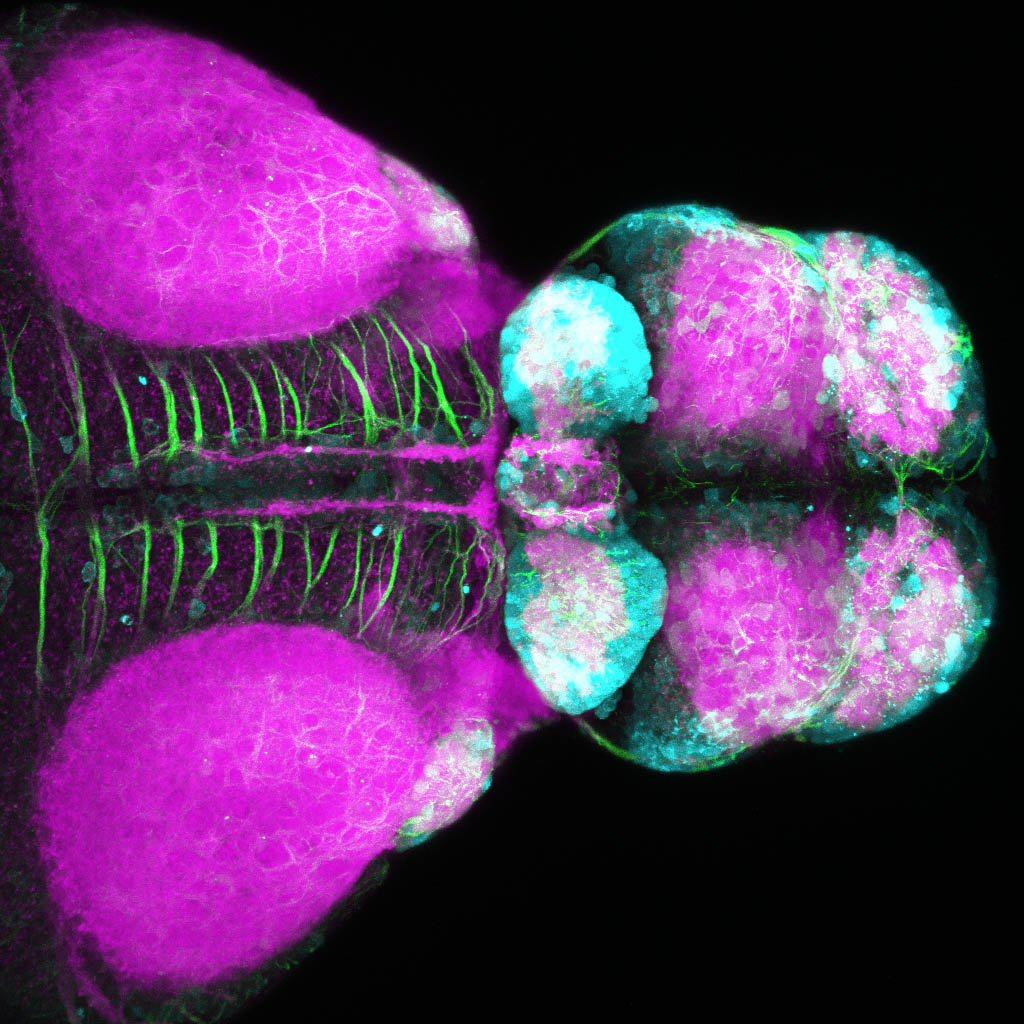
Glutamatergic neurons in the zebrafish forebrain
by Kate Turner
Glutamatergic neurons in the dorsal forebrain. Confocal micrograph of a 4 day old transgenic zebrafish viewed from a dorsal aspect. Glutamatergic neurons are shown in cyan, neuropil in magenta, and axonal tracts are labelled green. Neurons in the paired habenula nuclei in the dorsal diencephalon are strongly glutamatergic along with neurons in the olfactory bulbs, telencephalon and pretectum.
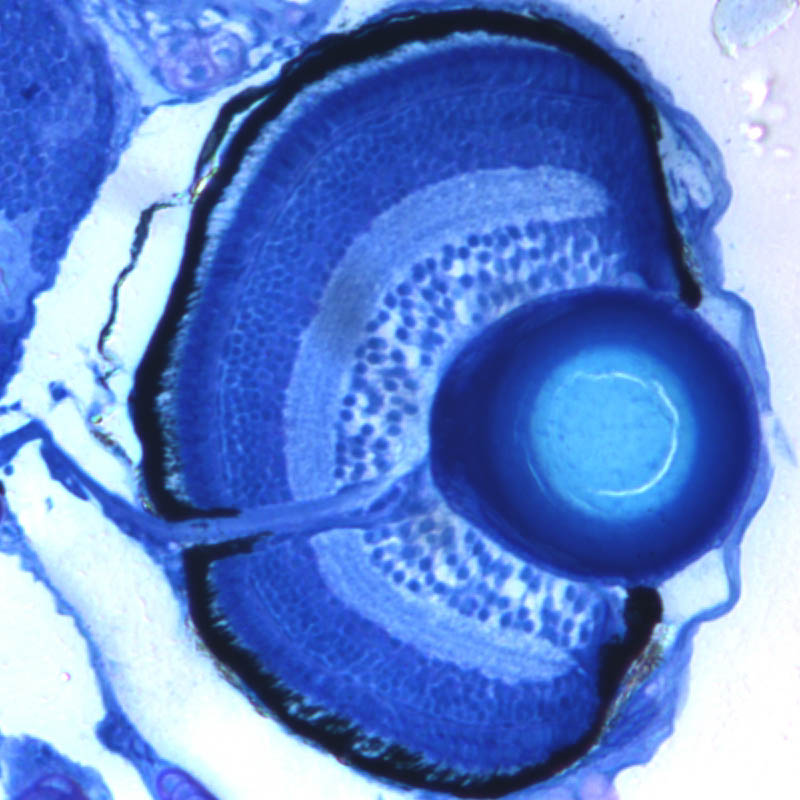
Zebrafish retina at 3 dpf
by Kate Turner
Transverse section showing the cytoarchitecture of the eye

Brn3a:GFP lights up the habenula
by Kate Turner
Dorsal view of a 4dpf Tg(Brn3a:GFP)embryo. labelled with anti-GFP and anti-tubulin antibodies.

Newly born retinal ganglion cells
by Kara Cerveny
Newly born retinal ganglion cells are marked by the ath5::GFP transgene. All nuclei are stained red with sytox orange.

Stem cells and progenitors in the eye
by Kara Cerveny

Cranial nerves
by Kate Turner
Dorsal view of an isl1:GFP larva labelled with anti-GFP(green) and anti-tubulin(red).

Absence of dividing cells in a wound
by Katie Woolie
Alexa-phalloidin and anti-H3 labelled fish embryo showing absence of dividing cells in a wound

Neurons in the left habenula
by Isaac Bianco

Section
By Leo Valdivia
Transverse section of brain and eyes of a 3 days post fertilization atoh7:GFP transgenic zebrafish larvae. The GFP expression of the transgene identifies the retinal ganglion cell layer of the retina and their axons that project to the processing centre in the brain. Nuclei are counterstained with DAPI.

Zebrafish posterior lateral line development
By Leo Valdivia
The lateral line is a sensory system in fish composed of neuromast cells, which detect vibrations in the water. This image shows the development of the lateral line in a 55 hour old zebrafish embryo containing a mutated form of the gene lef1. The lateral line is produced by a cluster of migratory cells called the posterior lateral line primordium (PLLP), and lef1 is a Wnt-pathway transcription factor required to maintain proliferation in the PLLP progenitor pool as the primordium migrates along the tail, periodically depositing neuromasts (green). The nuclei (DNA storage site) of cells of the PLPP are seen in red.

Spiral Eye
By Leo Valdivia
Pseudocolored confocal maximum projection of a 30 hours post fertilization double transgenic zebrafish retina. The transgene vsx2:GFP labels multipotent progenitors (in red), and atoh7:RFP marks committed cells during retinal neurogenesis (in green).
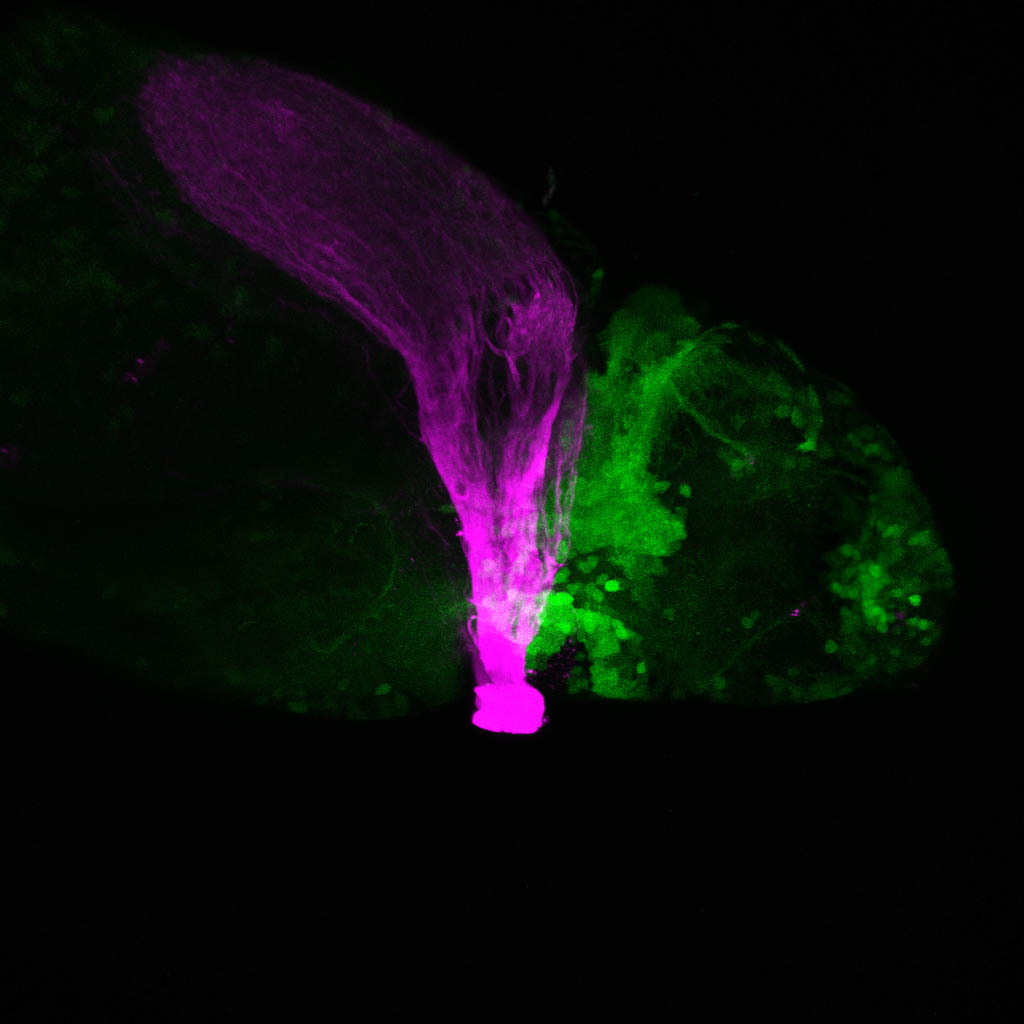
Where the optic tract meets the diencephalon
by Kate Turner
Lateral view of a double transgenic larval zebrafish with the optic tract labeled in magenta and cells in the diencephalon labelled in green showing the intermingling of these cells with the retinofugal pathway.

lhx5:GFP:atoh7:RFP
by Kate Turner
Dorsal view of a double transgenic larval zebrafish labelled with anti-GFP(green) and anti-RFP(magenta) showing GFP+ neurons in the forebrain particularly the olfactory bulbs and RFP+ Retinal Ganglion Cell axons terminating in the optic tectum.

Fish Head
by Lucas Roth

Lots of zebrafish (colour)
by Lucas Roth

Zebrafish eye
by Lucas Roth

Pineal (blue), left sided parapineal (green) and habenular neuropil (red)
by Isaac Bianco

A Salmonid embryo
by Author unknown
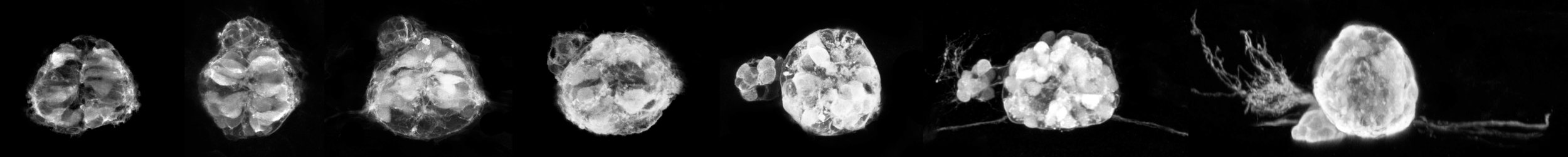
Zebrafish parapineal development
By Ingrid Lekk
Time series showing the migration of the parapineal in Tg(FoxD3:GFP;flh:GFP) embryos labelled with anti-GFP.
During zebrafish brain development, a small group of cells delaminates from the pineal organ and migrates to the left as a rosette to form a parapineal, which sends out unilateral axon projections to habenular nuclei. The parapineal conveys photoreceptive information to the pineal gland, which secretes melatonin to regulate sleeping patterns. Formation and migration of the parapineal underlies the development of left-right asymmetries in the dorsal diencephalon.

Neurons in the right habenula
by Isaac Bianco

Dopaminergic neurons in the zebrafish brain
by Kate Turner
Lateral view of a 3dpf slc6a3:GFP larva labelled with anti-GFP.
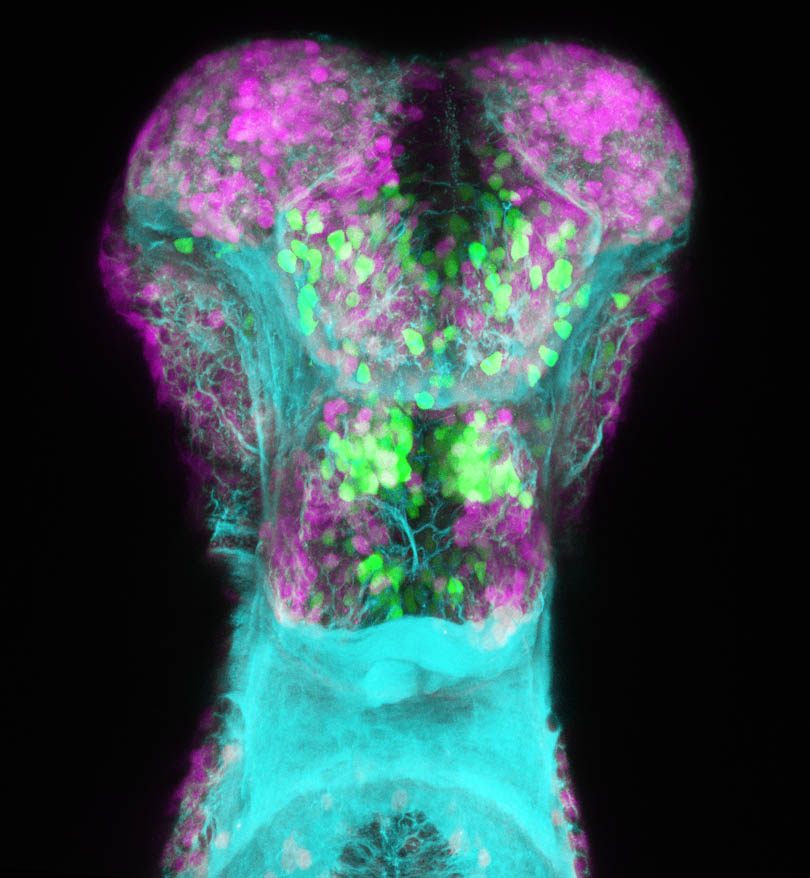
Glutamatergic and GABAergic neurons in the subpallium
by Kate Turner
vgult2a:DSRed(magenta), anti-GABA (green) and anti-tubuiln(cyan) in the subpallium of a 4dpf zebrafish larva..

Dopaminergic neurons in the zebrafish brain
Lateral view of a 3dpf slc6a3:GFP larva labelled with anti-GFP and anti-SV2.

lhx2a:GapYFP
by Kate Turner
Olfactory sensory neurons and mitral cell axons labelling the olfactory projectome in 4dpf larval zebrafish. Transgenic line from the excellent Miyasaka et al(2009) paper.

Dorsal view of the forebrain of a zebrafish larva showing axons (blue) and neuropil (pink).
by Jen Cook

Frontal view of the forebrain of a zebrafish larva showing axons (blue) and neuropil (pink).
by Jen Cook

Lateral view of the forebrain of a zebrafish larva showing axons (blue) and neuropil (pink).
by Jen Cook
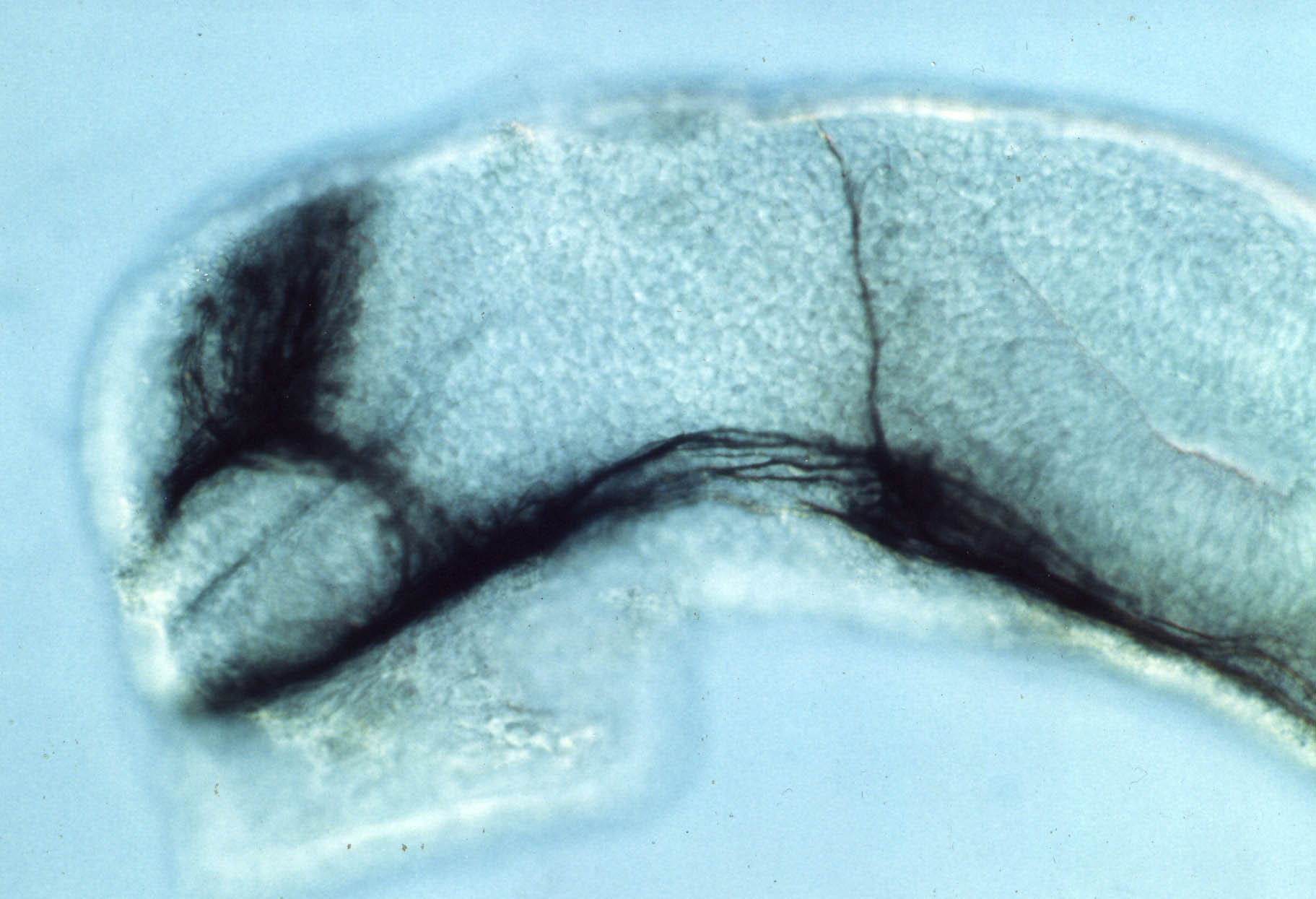
Anti-tubulin labelled axons in the fish brain
by Steve Wilson

Lateral view of a living fish embryo
by Steve Wilson

Mitral cells of the olfactory bulb in Tg(tbr:YFP)
by Kate Turner
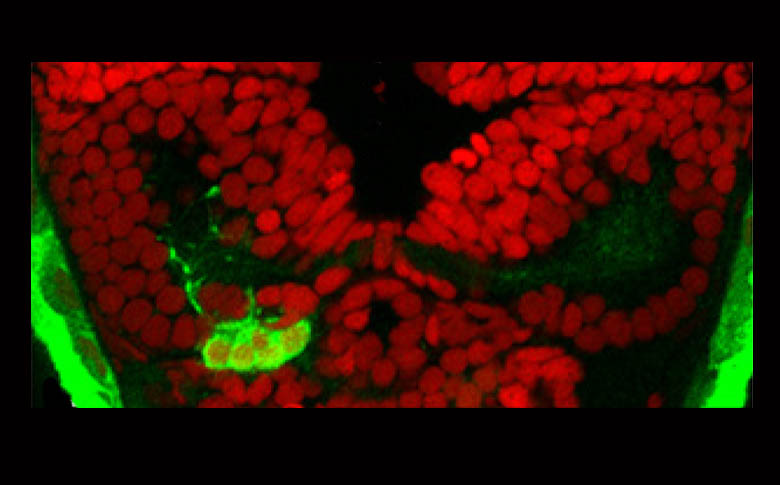
Parapineal(green) with ToPro(red).
by Myriam Roussigné
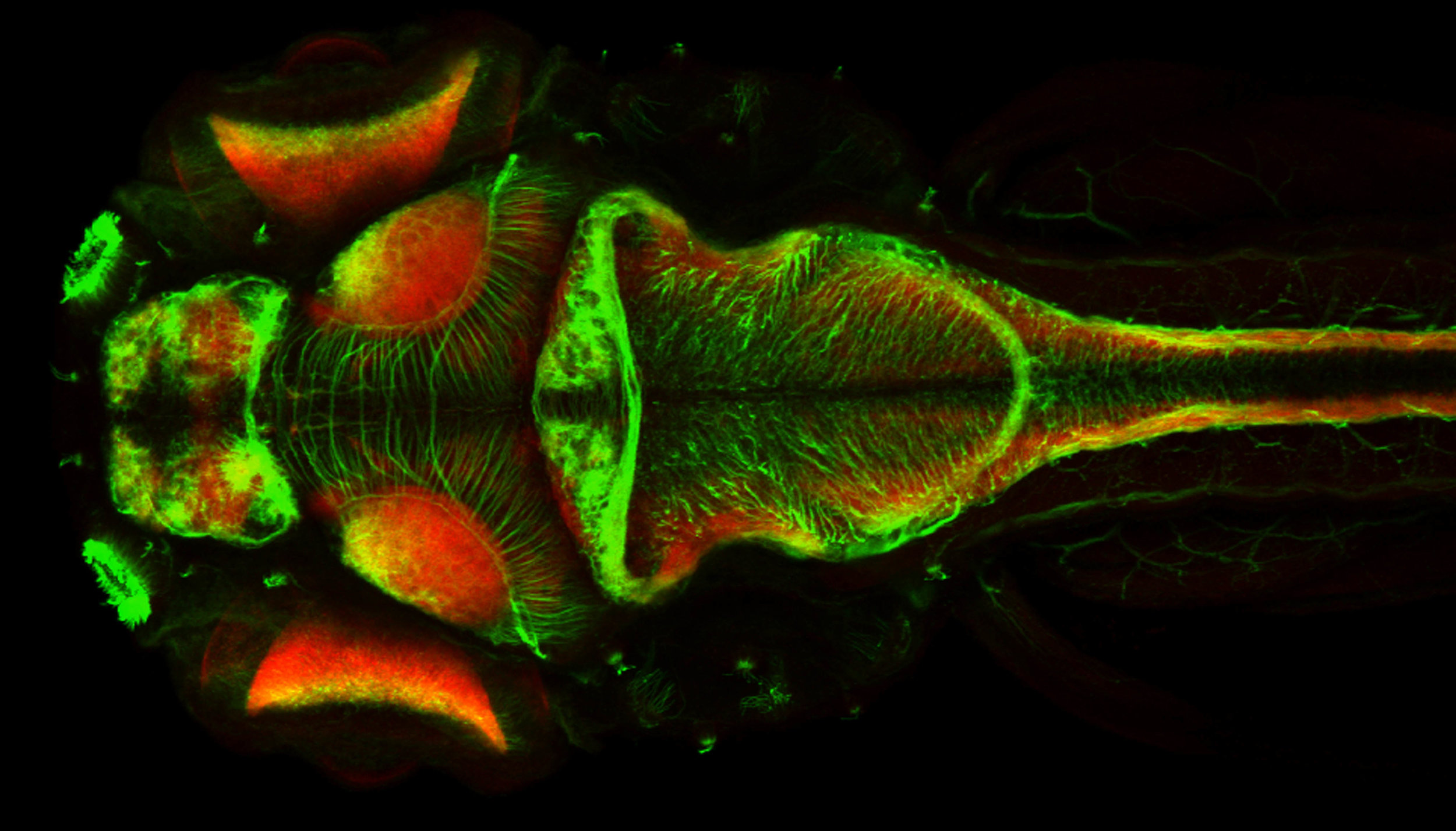
Neuronal tract labelling (green) and neuropil (red) in 4dpf brain, dorsal view.
by Jay Patel

Evaginating optic vesicles
By Florencia Cavodeassi

Cancer cells
Cancer cells apically popping out of the zebrafish embryonic epithelium.
By Masa Tada

Tada lab

Tada lab

Tada lab

Tada lab

Tada lab

Tada lab

Mirror symmetric image of cavefish embryo
By Monica Folgueira
Black and white confocal micrograph of a cavefish embryo at around 5 days post-fertilization. The embryo has been stained with an antibody that marks a calcium binding protein (calretinin). This highlights different neuronal types and their processes in the nervous system. This staining also labels taste buds, which can be seen as dark black dots located around the mouth and running along the body.
Cavefish live in dark caves and thus their natural habitat has no light. As a result adult cavefish are blind. Their eyes do develop and are still present at the embryonic stage shown in this image, but they will naturally degenerate during later stages.
The same image has been reflected to create a symmetrical mirror image.
This image won a Wellcome Image Award.

Frontal view of cavefish and zebrafish embryos
By Monica Folgueira
Confocal micrograph of a cavefish (left) and a zebrafish (right) embryo viewed from the front showing the head.
Both embryos are stained with fluorescent antibodies; one targets a calcium binding protein (calretinin) shown in yellow and blue, the other marks a tight junction component (zona occludens 1) shown in pink.
Calretinin labels different cell types in the brain and sensory organs. Taste buds are labelled around the mouth in both embryos (yellow in the cave fish and blue in the zebrafish). Zona occludens 1 is used to reveal tissues with an epithelial organization, such as the ventricular surface in the brain in both fish.
Researchers can learn a lot about development by comparing different organisms and the cavefish is often used in studying evolutionary development. Cavefish live in total darkness so adult cavefish are blind. The eyes develop during early embryonic stages but degenerate later on.

Cavefish Zebrafish
By Monica Folgueira
Confocal micrograph of a cavefish (left) and a zebrafish (right) embryo viewed from the front showing the head.
Both embryos are stained with fluorescent antibodies; one targets a calcium binding protein (calretinin) shown in yellow and blue, the other marks a tight junction component (zona occludens 1) shown in pink.
Calretinin labels different cell types in the brain and sensory organs. Taste buds are labelled around the mouth in both embryos (yellow in the cave fish and blue in the zebrafish). Zona occludens 1 is used to reveal tissues with an epithelial organization, such as the ventricular surface in the brain in both fish.
Researchers can learn a lot about development by comparing different organisms and the cavefish is often used in studying evolutionary development. Cavefish live in total darkness so adult cavefish are blind. The eyes develop during early embryonic stages but degenerate later on.



































































































