The African turquoise killifish (nothobranchius furzeri) is a promising new animal model for biogerontology due to its short lifespan of six months and its ability to halt their development (Kim, Nam & Valenzano, 2016). Due to their unique environment, temporary savannah pool in Africa and Neotropics (Cellerino, Valenzano and Reichard, 2016), the embryos can halt their development when the pools dry up and continue developing during wet season. Further, the killifish are interesting to investigate differences between sexes, as the females are smaller and pale and the males are colourful and bigger.
Unlike mammals, killifish have the ability to regenerate their central nervous system (Cellerino, Valenzano and Reichard, 2016) however they are unique that with increase age, the killifish show mammal-like ageing hallmarks with a decrease ability to regenerate tissue (Van houke et al., 2021). This makes it a great animal model to understand the processes of regeneration in young age and the changes in older age that cause ageing.
Taking all of this together, the killifish is a very promising model for biogerontology to understand the processes of tissue regeneration and ageing. We are working on characterising the killifish retina throughout the life course to identify mechanisms of ageing. Furthermore, we have tested many antibodies in the killifish which results can be found below.
Characterising the killifish retina throughout the life course to identify mechanisms of ageing. We will employ visual function assays, genetics, histology, single cell multi-omics technologies and spatial biology approaches to identify and validate molecular mechanisms driving cellular dysfunctions and degenerations during retinal ageing.
The Retina
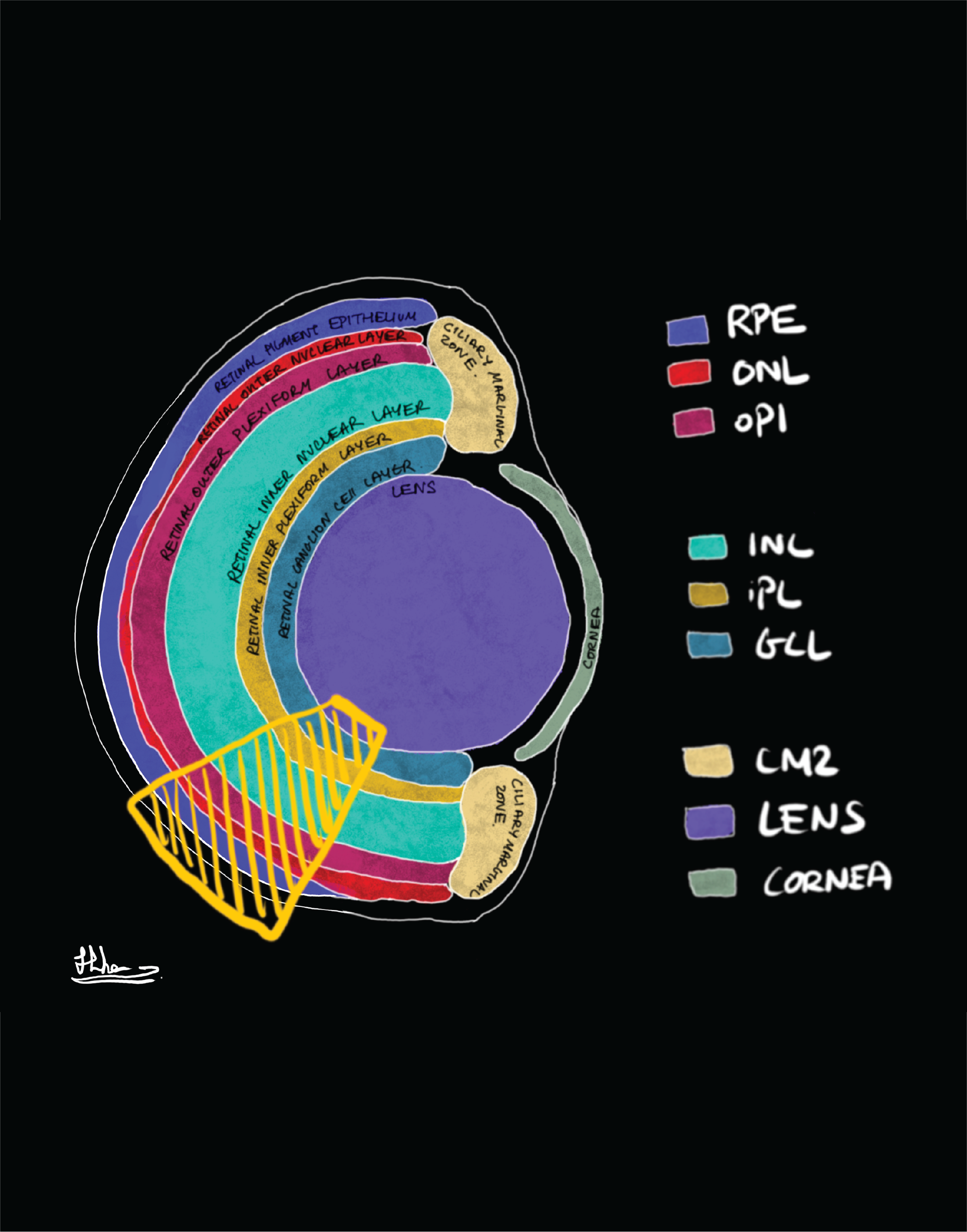
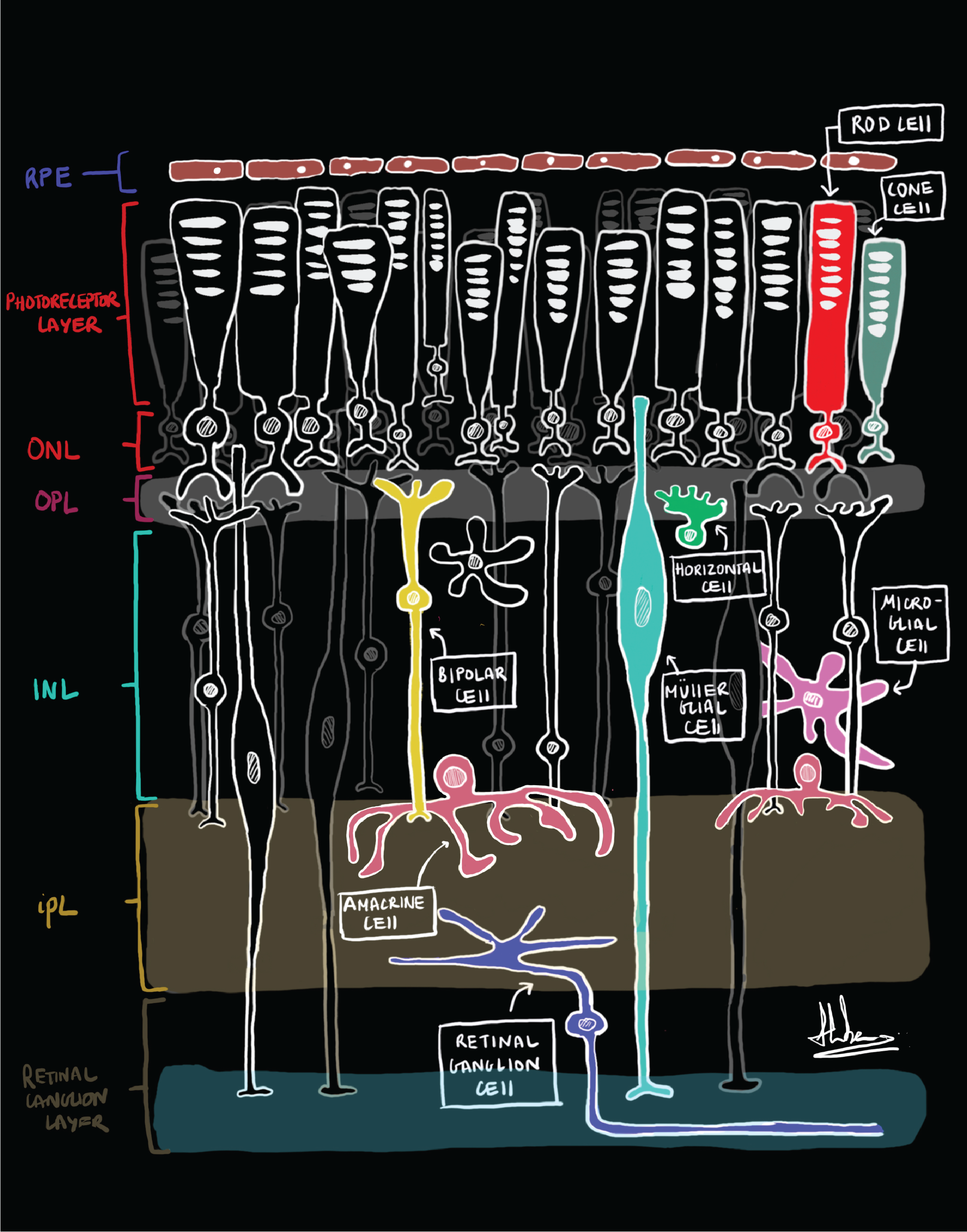
For the brain, the eyes are the window to the world. Light enters the eye through the lens and shines onto the retina at the back of the eye. The retina is a highly organised tissue which consists of six interconnected layers of neurons which convey light signals to the brain. Due to its highly organised nature, the retina give the possibility to unravel neural circuits and its cellular and molecular functions. The six retinal layers are, starting apically are the photoreceptor layer, the outer nuclear layer, the outer plexiform layer, the inner nuclear layer, the inner plexiform layer and the ganglion cell layer. The photoreceptors connect to the bipolar cells, which connect to the ganglion cells. The axons of the ganglion cells for the optic nerve and pass on the signal to the brain. Amongst those cells are horizontal cells connecting the different photoreceptors, and the amacrine cells which interconnect the horizontal and ganglion cells (Salman, McClements and MacLaren, 2021). Müller glia (MG) are the principal glia cells, stretching through all of retina layers.
Retinal Schematics of layers (left) and cells (right): Credit to the undergraduate student Hiba Noor
retinal cell types described
Click on the different retinal cell types below for a description of the cells and a list of antibodies that label them in killifish.
Disclaimer: We followed two immunohistochemistry protocols with both NaCit and Tris-HCl antigen retrieval. For everything we used standardised solution 1/100. We did however not validate many of the antibodies for specificity in killiifish, zebrafish in mutants or western blot.
People working on this
RELEVANT LITERATURE
Cellerino A, Valenzano DR, Reichard M. 2016. From the bush to the bench: the annual Nothobranchius fishes as a new model system in biology. Biological Reviews. 91:511-533.
Kim Y, Nam HG, Valenzano DR. 2016. The short-lived African turquoise killifish: an emerging experimental model for ageing. Disease Models & Mechanisms. 9:115-129.
Salman A, McClements ME, MacLaren RE. 2021. Insights on the regeneration potential of Müller glia in the mammalian retina. Cells. 10:1-12.
Van houcke J, Mariën V, Zandecki C, Vanhunsel S, Moons L, Ayana R, Seuntjens E, Arckens L. 2021. Aging impairs the essential contributions of non‐glial progenitors to neurorepair in the dorsal telencephalon of the Killifish Nothobranchius furzeri. Aging Cell. 20:1-18.