PROLIFERATE, DIFFERENTIATE OR DIE?
MAKING DECISIONS IN THE DEVELOPING EYE
Kara Cerveny, Florencia Cavodeassi and Steve Wilson
The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. (2010)
Nearly 40 years after US President Richard Nixon declared 'war on cancer', researchers around the world are still trying to understand how tumors form and grow. New insight into how cells can be prevented from becoming cancerous is found in an unlikely place - the eyes of zebrafish.
Zebrafish are small striped fish commonly sold in pet stores, and are valued for their hardy nature. For scientists, zebrafish are model organisms that can be used to reveal new clues about all things biological including brain organization, immunology, cancer, and developmental diseases.
Zebrafish eyes, like the rest of their bodies, grow continuously. Each eye grows in a very controlled pattern -- new cells are added from a specialized region that encircles the edge of the camera-like part of the eye that senses light (the retina). In this way, the eye grows much like a tree, adding annular rings of new cells that must integrate into the existing tissue. Your eyes are different. They are the same size from the day you?re born until the day you die.
The longer a tissue continues to grow, the more likely its cells are to acquire cancer-like properties. Continued growth requires specific embryonic-like cells with unlimited growth potential, called stem cells. Specialized regions that house these stem cells and provide a continuous source of new cells are called stem cell niches. We can examine how tissues regulate their growth (proliferation) by studying accessible stem cell niches, like those in the eyes of zebrafish.
In this study, we analysed mutant fish whose eyes failed to grow. Although these fish have small eyes, their stem cells can, counter-intuitively, over-proliferate. The failure of the eyes to grow happens because the abnormally proliferating cells usually die. From a series of experiments, we learned that mature nerve cells (neurons), adjacent to the retinal stem cell niche, secrete signals that regulate the number of new cells produced. This new finding tells us that the environment surrounding a dividing cell may be just as important as its own genetic make-up.
In one key experiment, we transplanted mutant cells into normal (wild-type) eyes and asked how they behaved. We hypothesized that the mutant proliferating cells might form a small tumor in the host eye or be forced to die. Neither happened. Instead we found that the mutant cells behaved like their wild-type counterparts and contributed new neurons to the growing eye. This surprising result along with other data from our study suggests that the environment can guide dividing cells to differentiate into neurons.
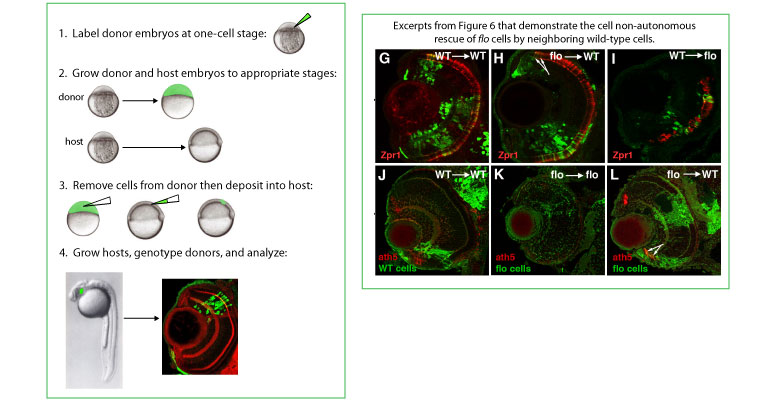
GENERATION OF MOSAIC EYES BY CELL TRANSPLANTATION IN ZEBRAFISH
On the left, a schematic illustrates how we generate eyes that contain both flo and wild-type cells. Above, several panels from our paper show that local differentiating and/or mature neurons influence flo cells to stop cycling and differentiate. Representative frontal sections of eyes at various ages labelled with antibodies (Zpr1) or antisense RNA (ath5) as shown bottom left. Zpr1 recognizes cone photoreceptors and Ath5 marks differentiating CMZ cells. GFP labeled cells from either wild-type (G, I, J) or flo (H, K, L) donor embryos were transplanted into wild-type (G,H, J, L) or flo (I, K) hosts. The arrows in H and L point to flo mutant photoreceptors or ath5+ cells emerging from the CMZ in wild-type eyes, respectively.
The mutant we studied is named flotte lotte (flo for short). We compared how quickly flo and wild-type eye cells divide, and found that flo mutant cells divide more slowly. Because flo eye cells divide slower than normal, they eventually activate a cell-cycle checkpoint that prompts them to commit suicide. Surprisingly, flo cells in a wild-type environment still cycle slowly, but instead of dying, they survive and differentiate into functional neurons. Instead of triggering death to remove the mutant cells (which are dividing aberrantly and could potentially contribute to cancerous growth), the environment removes them by coaxing them to stop dividing and differentiate into neurons. This is an attractive hypothesis for multiple reasons. It provides one possible explanation for why we find neurons in all vertebrates (including humans) that contain evidence of a history of cell division defects such as an abnormal number of chromosomes. It also provides an explanation for the organization and self-limiting behavior of the zebrafish retinal stem cell niche.
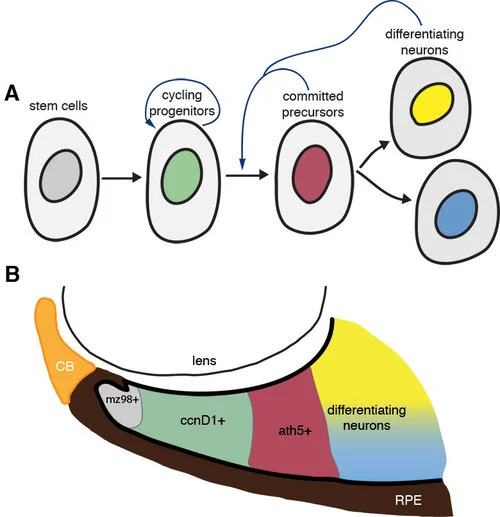
Our model suggests that the local differentiated environment adjacent to the CMZ pushes cycling retinal stem cells towards differentiation.
A) Cycling progenitors receive extrinsic feedback from surrounding cells to transition from proliferation to differentiation. Retinal stem cells at the periphery of the eye give rise to proliferating progenitors, the behaviour of which is regulated by intrinsic (cell-cycle) and extrinsic (environmental) cues that determine the probability that these cells will stop cycling and differentiate. Although flo cells have intrinsically compromised cell cycle kinetics, they are able to respond to wild-type extrinsic signals that promote their transition from proliferation to differentiation. B) The spatial organization of the CMZ: putative stem cells (mz98+), cycling progenitors (ccnD1+), committed precursors (ath5+), and differentiating neurons are sequentially arranged such that dividing cells inevitably come into contact with differentiating neurons as they move centrally from the CMZ; we suggest these differentiating cells encourage the dividing cells to enter their final cell cycle and differentiate (A).
Our model suggests that the local differentiated environment adjacent to the CMZ pushes cycling retinal stem cells towards differentiation. A) Cycling progenitors receive extrinsic feedback from surrounding cells to transition from proliferation to differentiation. Retinal stem cells at the periphery of the eye give rise to proliferating progenitors, the behaviour of which is regulated by intrinsic (cell-cycle) and extrinsic (environmental) cues that determine the probability that these cells will stop cycling and differentiate. Although flo cells have intrinsically compromised cell cycle kinetics, they are able to respond to wild-type extrinsic signals that promote their transition from proliferation to differentiation. B) The spatial organization of the CMZ: putative stem cells (mz98+), cycling progenitors (ccnD1+), committed precursors (ath5+), and differentiating neurons are sequentially arranged such that dividing cells inevitably come into contact with differentiating neurons as they move centrally from the CMZ; we suggest these differentiating cells encourage the dividing cells to enter their final cell cycle and differentiate (A).
Current and future experiments in our lab will try to figure out the exact molecules that drive flo cells to differentiate in a wild-type environment. We are also interested in determining if particular types of mutants, e.g., classes of mutants with similar cell cycle defects, are more susceptible to environmental rescue than others.
For more information check out a synopsis of our paper in Development or contact us directly.
This work was supported by a grant from the MRC and a post-doctoral fellowship from the Damon Runyon Cancer Research Foundation. Future work on this project is funded by Cancer Research UK.