MIGRATION ALONG THE ZEBRAFISH LATERAL LINE: HOMEOSTASIS BOUND
Leonardo Valdivia, Rodrigo Young and Steve Wilson
Lef1-dependent Wnt/β-catenin signalling drives the proliferative engine that maintains tissue homeostasis during lateral line development
During embryo development complex processes are coordinated in order to guide the formation of tissues and organs of the body. Organs and tissues are composed of different cell types which are sometimes specified (instructed to become a certain cell type) far from they will finally locate and become functional. Therefore the process of collective cell migration, where groups of cells move together as a whole, is fundamental for embryo development.
One great advantage of using zebrafish is that the embryos are transparent allowing visualisation of individual cells as the embryo develops. In our study we used zebrafish to investigate the biological basis of the tissue homeostasis (the ability of the tissue to maintain a stable equilibrium in cell number) in a collectively migrating group of cells: the posterior lateral line primordium (PLLP). The primordium is a cohesive cluster of cells that moves directionally from behind the head to the tip of the tail in the developing zebrafish embryo. Whilst migrating, the PLLP sequentially deposits groups of cells that will form several small volcano-shaped structures called neuromasts, which are the sensory organs of the lateral line system and allow fish to perceive water motion.
In this study we focus on understanding how the PLLP maintains its size as it migrates, even though it continuously loses cells as neuromast are deposited. A useful way to work out how a biological process works is to look at an organism in which this process does not happen correctly. We can work out which step of the process is missing, and in doing so elucidate the mechanisms behind the process happening correctly. To this end, we analysed a mutant fish in which the PLLP vanishes prematurely giving rise to a reduced number of neuromasts (Fig 1). We found out that in the mutant the PLLP becomes depleted of precursor cells, which are cells that proliferate in order to regulate growth. When the mutant PLLP runs out of precursor cells, deposition of cells as neuromasts terminates prematurely and the PLLP appears to run out of steam, stopping before it reaches the finish line at the tip of the tail. Our findings shed light on the role of the mutated gene in sustaining proliferation to maintain the primordium tissue homeostasis.
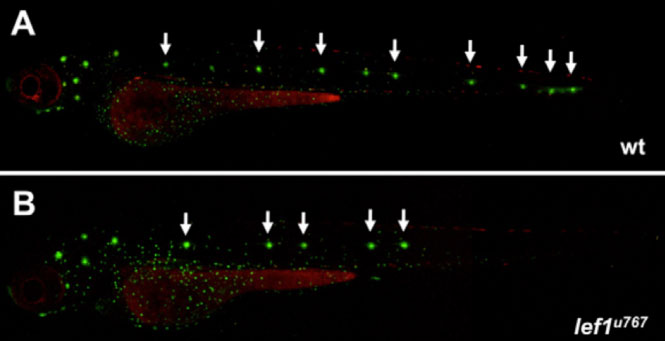
Figure 1. This picture shows the pattern of neuromasts in a normal and lef1 mutant larvae after 4 days of development. Lateral line neuromasts in zebrafish larvae are easily identifiable by different fluorescent dyes and assessed in vivo. Wild types show the expected distribution of seven or eight of these structures spanning the trunk and tail, when they are incubated in one of these dyes. The lef1 mutants have a reduced number of neuromasts, displaying only the first four or five and lacking the most caudal ones (B). The reagent used for staining in this case is DioC6, which emits in the green wavelength under fluorescence. Heads of the fish are to the left and tail to the right in both pictures.
The experiments
As a starting point in our investigation, we identified that a mutation in the lef1 gene was responsible for the phenotype. As we have described, the PLLP is a migrating collective of cells that move in one direction: from head to tail. The cluster has a front (called the leading edge) and back (trailing zone). In order to keep homeostasis as it systematically loses cells as neuromast deposits, cells of the leading edge actively proliferate. This adds cells to the trailing zone and in doing so feeds neuromast production, contributing to their overall size as they are deposited. This shows that the leading edge of the PLLP functions as a progenitor centre, a factory for the generation of new cells. Importantly, the leading edge is also the region of the PLLP where the lef1 gene is expressed.
Because the PLLP fades prematurely in the lef1 mutant, we asked if the mutation was affecting cell proliferation during migration. Using a stain to highlight cells undergoing proliferation we noticed a strong reduction of proliferating cells in the mutant primordium. This problem was restricted to the lef1 expression domain at the leading edge of the PLLP. As no new cells were generated to feed the neuromasts, the leading zone cells were soon used up as neuromast deposits (movies). Our results suggest that the progenitor zone is not able to keep up the rate as which it feeds the new arising neuromasts and so the system literally runs out of cells. We conclude that inability of the leading edge to proliferate is the main cause of the premature finishing of lateral line system in lef1 mutant.
Figure 2. The behavior of the wild type PLLP in the tip of the tail is reminiscent of what happen further rostrally in lef1 mutants. In (A), a three days old transgenic larva shows the normal pattern of neuromast (this transgenic line labels all the cells of the lateral line with a green fluorescent protein that is shown in gray in this figure). In (B), an inset of the most posterior neuromast from (A) is shown. Note the neuromast in close succession. In (C), you can see frames from a movie starting when the seventh neuromast is deposited, using the same transgenic line than (A). This show that, as it reaches the tip of the tail, the PLLP splits and simultaneously forms the terminal neuromasts with a few remaining cells subsequently dispersing similar to the lef1 mutant (compare with the movies)
What happens to the PLLP as it nears the end of its migratory route? How does the PLLP know to stop migrating at the tip of the tail in the wild-type embryo? We don't see a build up of cells in the tail, a clear indication that proliferation stops once the last neuromast is deposited. Further research showed that the premature end to migration in mutants resembles what happens to the wild-type primordium when it reaches the tip of the tail. We conclude that switching off expression of the lef1 gene is the endogenous mechanism by which the wildtype primordium stops migrating, reducing cell proliferation as it reaches the tip of the tail and eventually fading, just like the premature end to the lef1 mutant primordium (Fig 2).
Why is this study important for the wider scientific community and society in general? We propose a novel mechanism to regulate patterning by controlling cell proliferation in a migrating group of cells. Lef1 is a component of the Wnt pathway, a major genetic pathway involved in development. The function of Wnt signalling through lef1 could be important for other collective cell growing/migrating systems. For instance, we also observed a reduction/absence of fins at post-embryonic stages in mutants, which could be also due to a failure in fin growth. This lead us to hypothesise that there may be a conserved role for Lef1-mediated Wnt signalling at the heart of the mechanism controlling proliferation in different tissues. Additionally, as lef1 expression is known to be enhanced in some cancer cell lines and to mediate invasive properties of these cells through metastasis, it will be interesting to determine if this behaviour is related to the proliferative role of lef1.
If you have any more questions about this research contact Leo , Rodrigo or Steve Wilson.
This work was supported by the Wellcome Trust, the Royal Society, and by a Marie Curie Incoming International Fellowship and travelling fellowships from The Company of Biologists
Movie1. Normal migration of the PLLP in a transgenic embryo expressing a fluorescent protein in the primordium (lateral view, anterior to the left). In this video, four neuromasts are deposited evenly by the primordium, which continues its migration.
Movie2. Curtailed migration of the PLLP in a transgenic lef1 mutant (lateral view, at a level comparable with movie1). In this video, only three neuromasts are deposited by the primordium, which then peters out, forms a long slender process and ceases to migrate.