Leila Romio and Steve Wilson
Original paper reference
Convergent extension movements and ciliary function are mediated by ofd1, a zebrafish orthologue of the human oral-facial-digital type 1 syndrome gene
Oral-facial-digital syndrome type 1 (OFD1) is a severe condition that occurs in 1:50,000-250,000 live births. The disease is caused by a defect in a gene called ofd1 that is carried on the X chromosome. We know that Ofd1 protein has a crucial role in development, because XY males inheriting the mutation have no Ofd1 and die before birth, whereas heterozygous XX females (who carry one mutant and one working copy of the gene) are born with several congenital defects: malformation of the face and mouth, abnormalities of the digits and malformation of the central nervous system. OFD1 syndrome often features polycystic kidneys, which is the main cause of death among patients and that can only be treated effectively by kidney transplant. Ofd genes are present in all vertebrate animals and this means that one can potentially model the disease in animals in which it is easier to study why developmental events go wrong than it is in humans. In this study, we elucidated the function of ofd1 during development by depleting Ofd1 protein during zebrafish embryogenesis, using morpholino (Mo) antisense reagents that inhibit the activity of the gene.
Ofd1 protein localises to basal bodies - these are structures inside cells from which arise cilia - tail-like projections from the cell surface. Recent studies have highlighted that many genes mutated in complex genetic syndromes produce proteins that localise in cilia or at their root. Cilia are present on almost every cell type in vertebrates, and can be motile or non-motile. Motile cilia are found on various structures during development including the embryonic node in mouse and Kupffer's vesicle (KV) in zebrafish. These are structures in which active motile cilia generate fluid flow towards the left side of the body and this is important for establishing the left-right placement of organs like the heart and liver. Cilia also mediate cerebrospinal fluid movement and respiratory tract mucous clearance. A class of cilia called primary cilia are generally not actively motile but are important for the transmission of signals from the outside to the inside of cells. For example those located on mammalian renal (kidney) epithelial cells sense renal tubular flow by bending, thus instigating signalling which maintains epithelial differentiation.
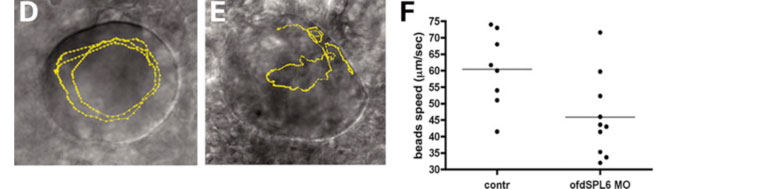
Figure 1. Cilia movement is disrupted when Ofd1 doesn't function: the yellow line shows the path of a single bead inside Kupffers' vesicle, A is a normal embryo, while B is an embryo where Ofd1 function is disrupted. The plot shows the difference in bead speed between normal and affected embryos
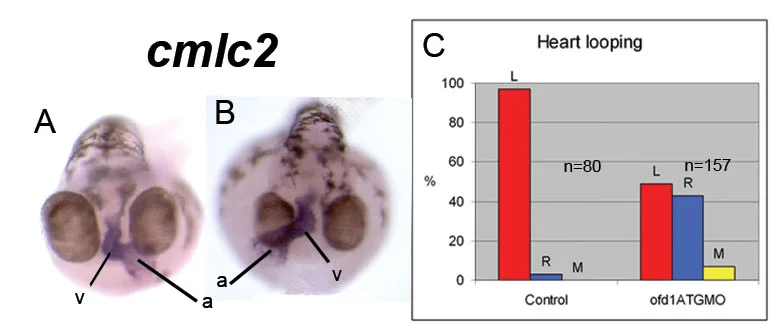
We have studied how zebrafish development is affected by partial depletion of Ofd1 protein, as happens in female patients. The main findings of our study are: Ofd1 depletion disrupts cilia motility. Although cilia are present they don't function correctly when Ofd1 function is disrupted. This is demonstrated by an experiment where we injected very tiny beads into Kuppfers' vesicle (a vesicular structure covered by motile cilia) and observed that cilia movement is disrupted in ofd1-depleted embryos compared to normal embryos (Figure 1). The consequence of this disruption is that some of the affected embryos show altered left-right patterning, that is, asymmetric positioned organs (the heart for example) can be found on either side of the body (Figure 2).
Figure 2. Heart laterality: A shows the normal postion of the heart (blue), B is an example of a laterality defect where the heart loops in the opposite direction. The plot shows the proportion of right/medial/left positioned hearts in normal embryos and embryos with disrupted Ofd1 function.
Ofd1 has a role in movements by which cells elongate the embryo during early development. Cell movements during the gastrulation phase of embryonic development are regulated by the so-called Wnt/PCP signalling pathway. When Ofd1 is not functioning correctly, these movements are disrupted and embryos are shorter than normal. We showed ofd1 has genetic interactions with two genes of the Wnt/PCP pathway, vangl2 and wnt11 (Figure 3). This means that mutations in two genes leads to a more severe developmental defect than in either alone.
Figure 3. This picture shows how ofd1 genetically interacts with vangl2 in gastrulation cell movements, resulting in shorter larvae: partial loss of ofd1 doesn't affect embryo length, but when there is also disruption to the vangl2 gene, this results in much shorter embryos.
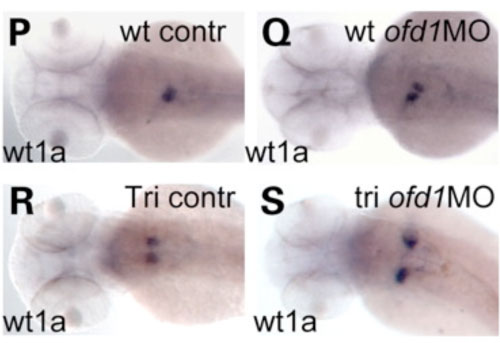
Ofd1 is required for fusion of the two primordia that normally merge at the midline to form the kidney. Embryos lacking functional ofd1 often have split kidneys due to a failure in migration of the cells that from this structure (Figure 4). We speculate this might be related to the other defects we observe in cell migrations during early development.
Figure 4. The pronephroic glomerulus (the precursor of the kidney) forms from two primordia that migrate towards the midline and fuse. In ofd1 compromised embryos, this process is impaired, and we observed the same phenomenon in mutant fish called trilobite (tri) that carry a mutation in vangl2. We speculate that this could be another consequence of disrupting Wnt/Pcp dependent cell migrations.
Overall, our study has helped to elucidate why mutations in ofd1 can cause severe problems in humans by demonstrating critical roles for the gene in cilia function and in cell movements during embryogenesis.
Further reading
- Faculty of 1000 appraisal
- ofd1 syndrome healthline link
- ofd1 syndrome webmd link
- ofd1 syndrome wiki link
This study was a collaboration between the groups of Derek Stemple at the Sanger Centre, Adrian Woolf at the Institute of Child Health at UCL and Steve Wilson also at UCL. Most of the support for the work was provided by the Wellcome Trust.