About
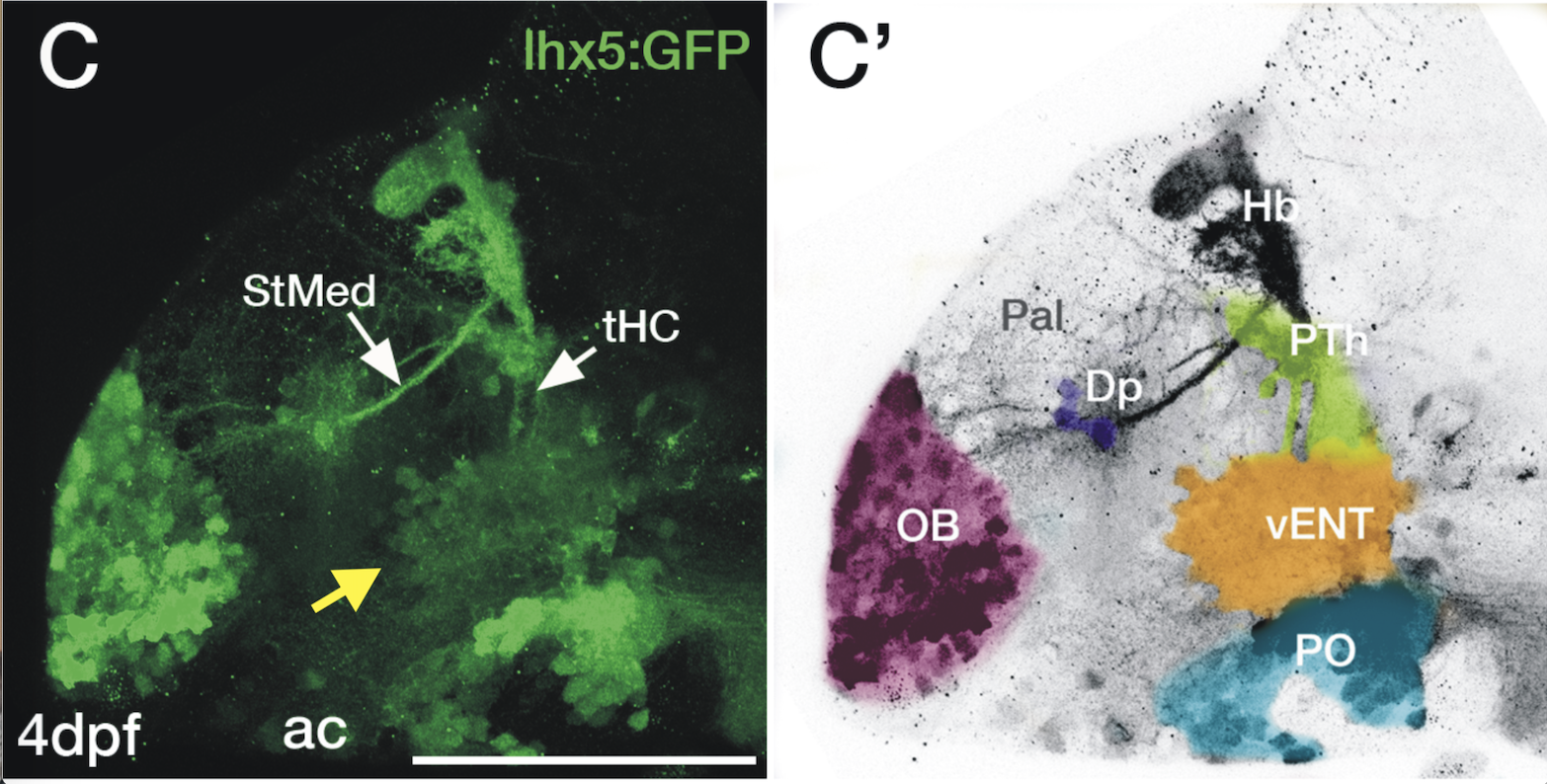
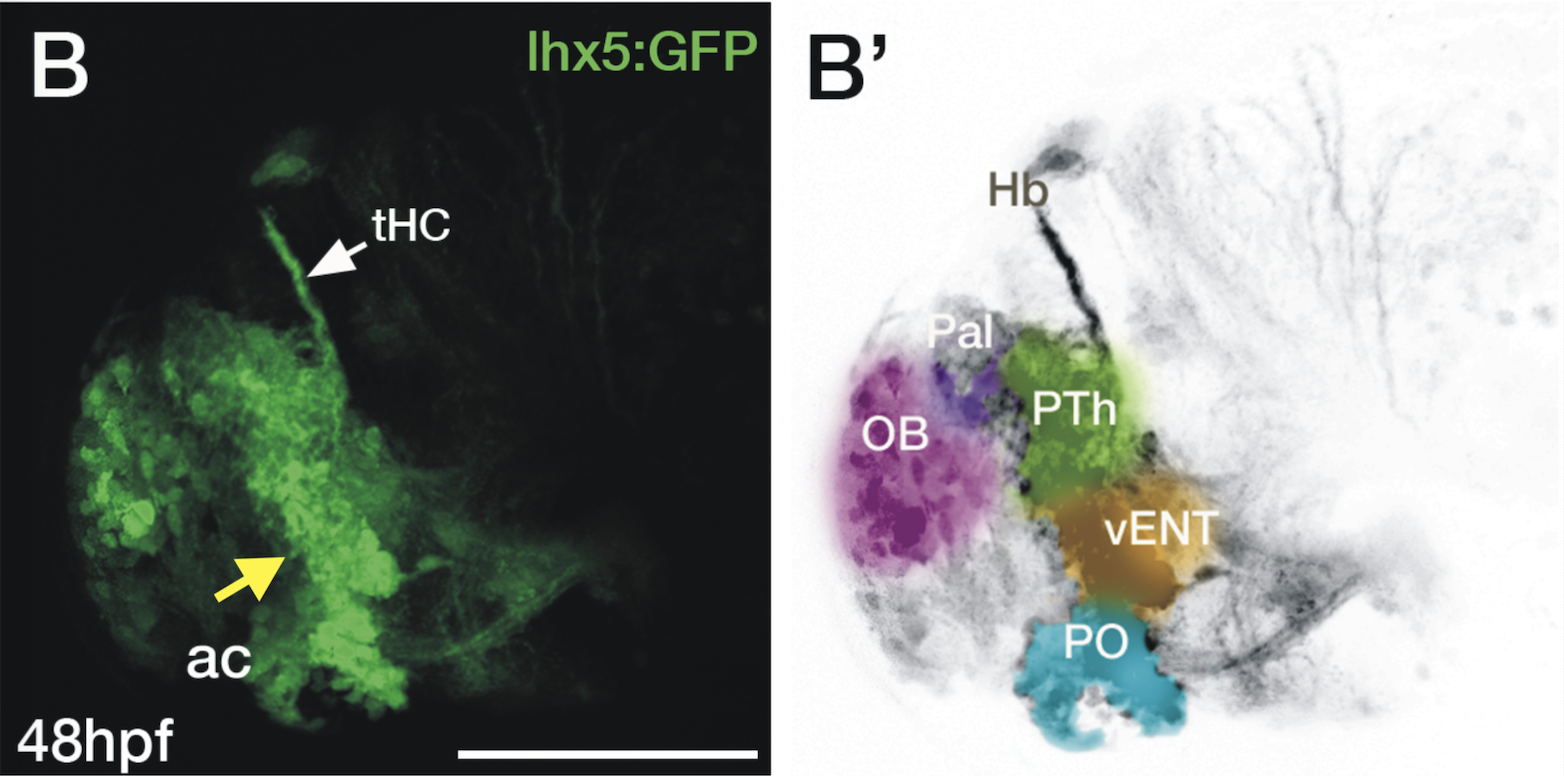
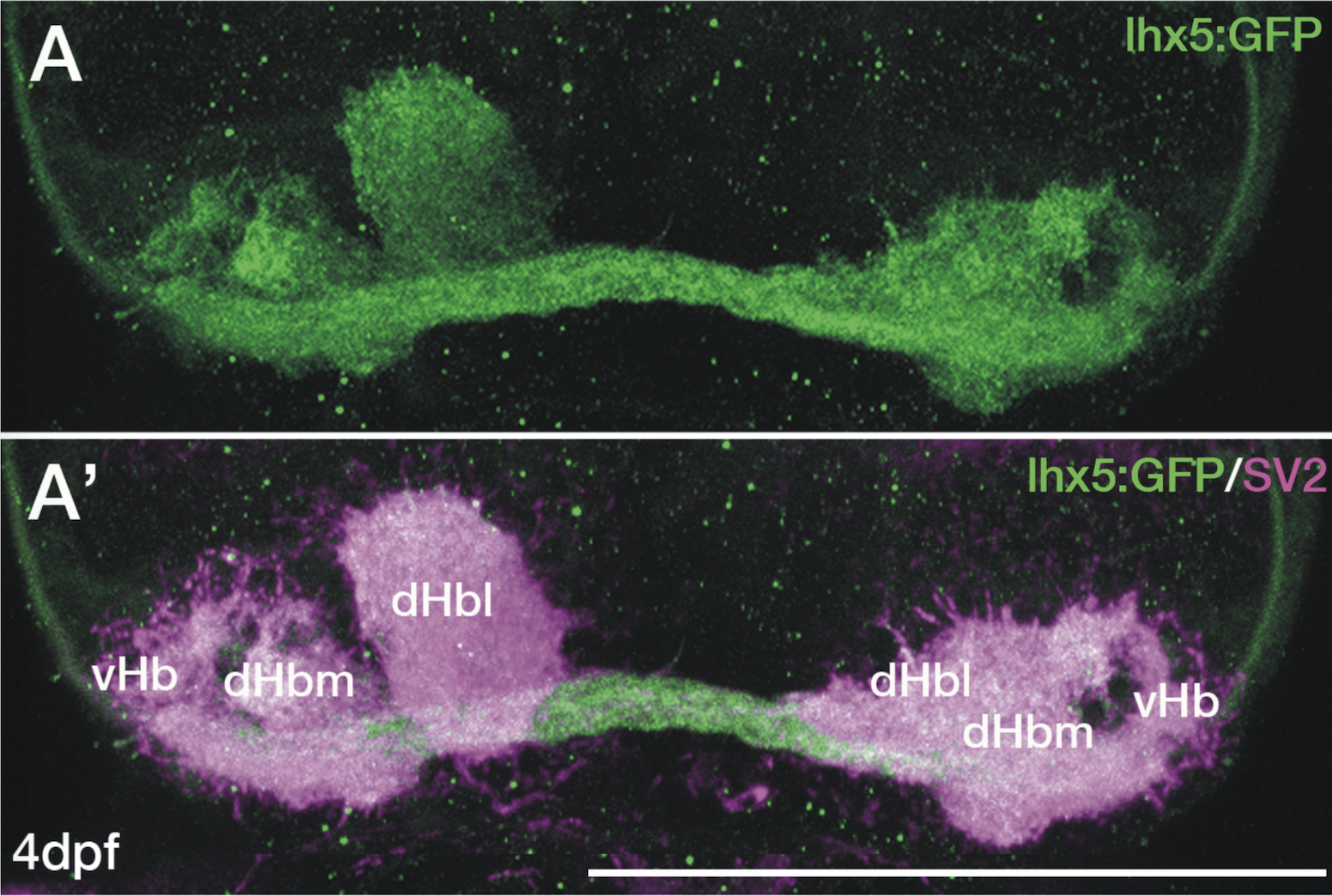
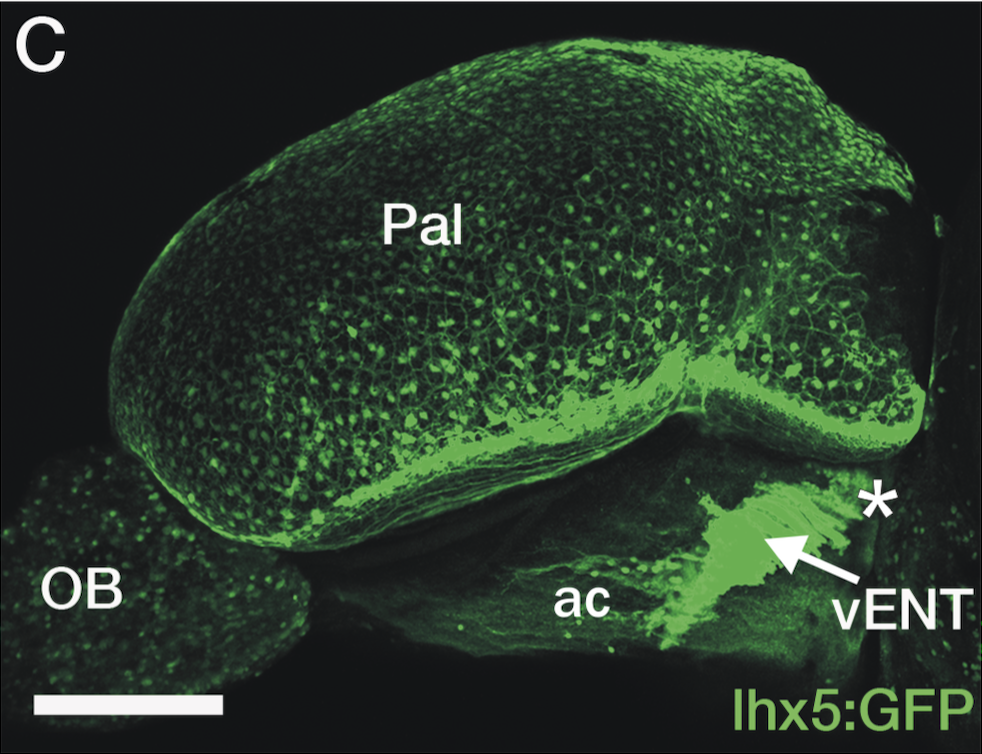
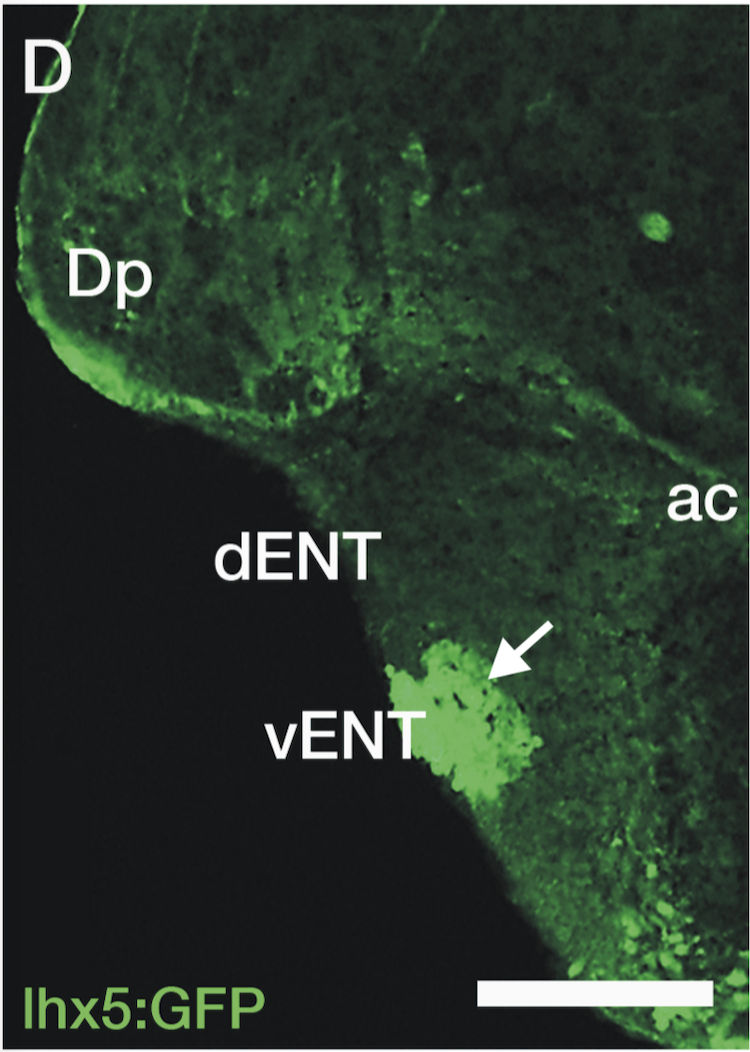
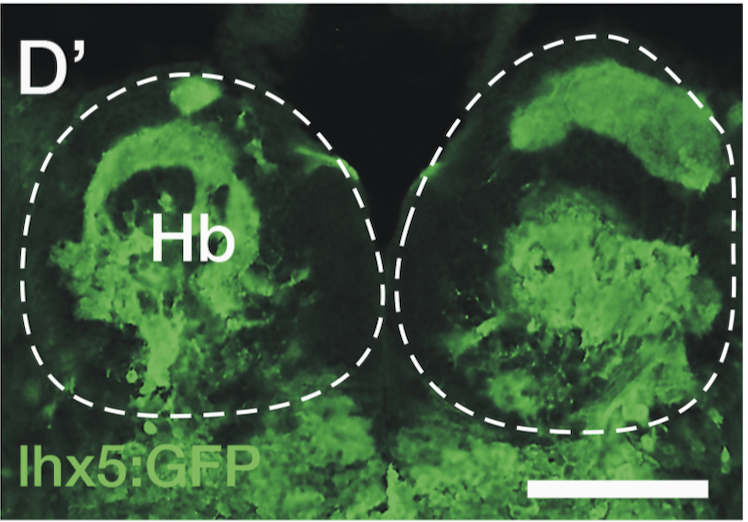
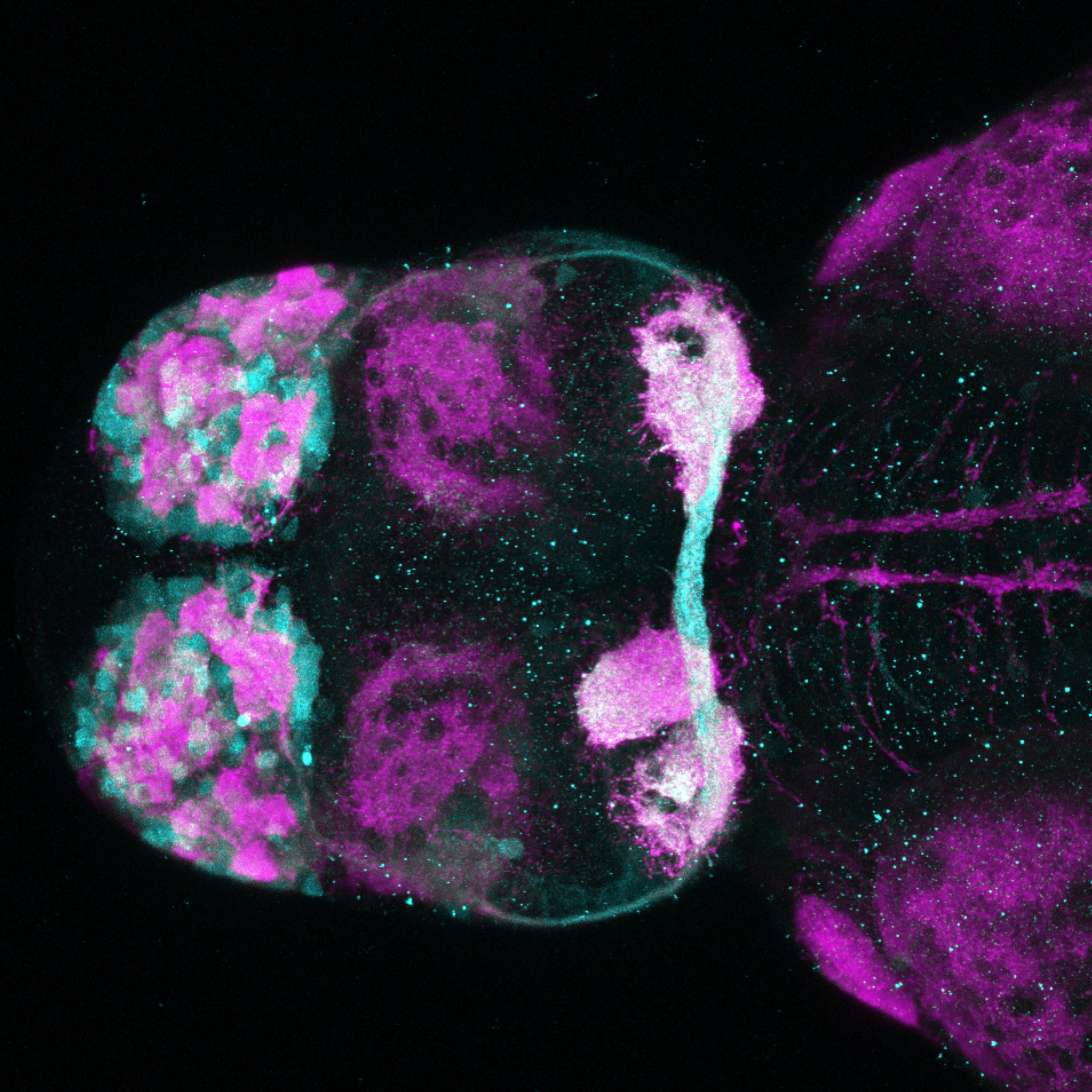
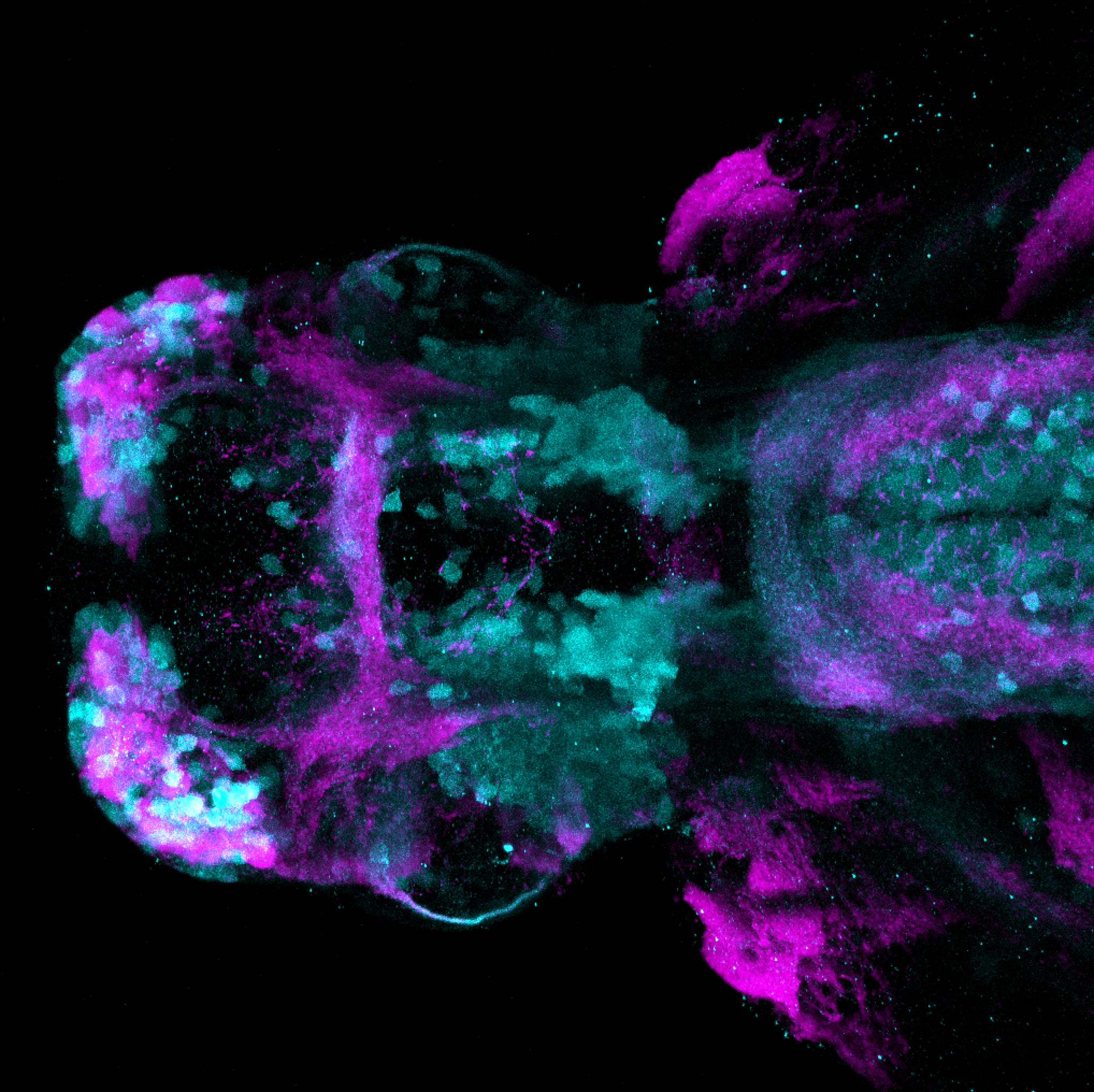
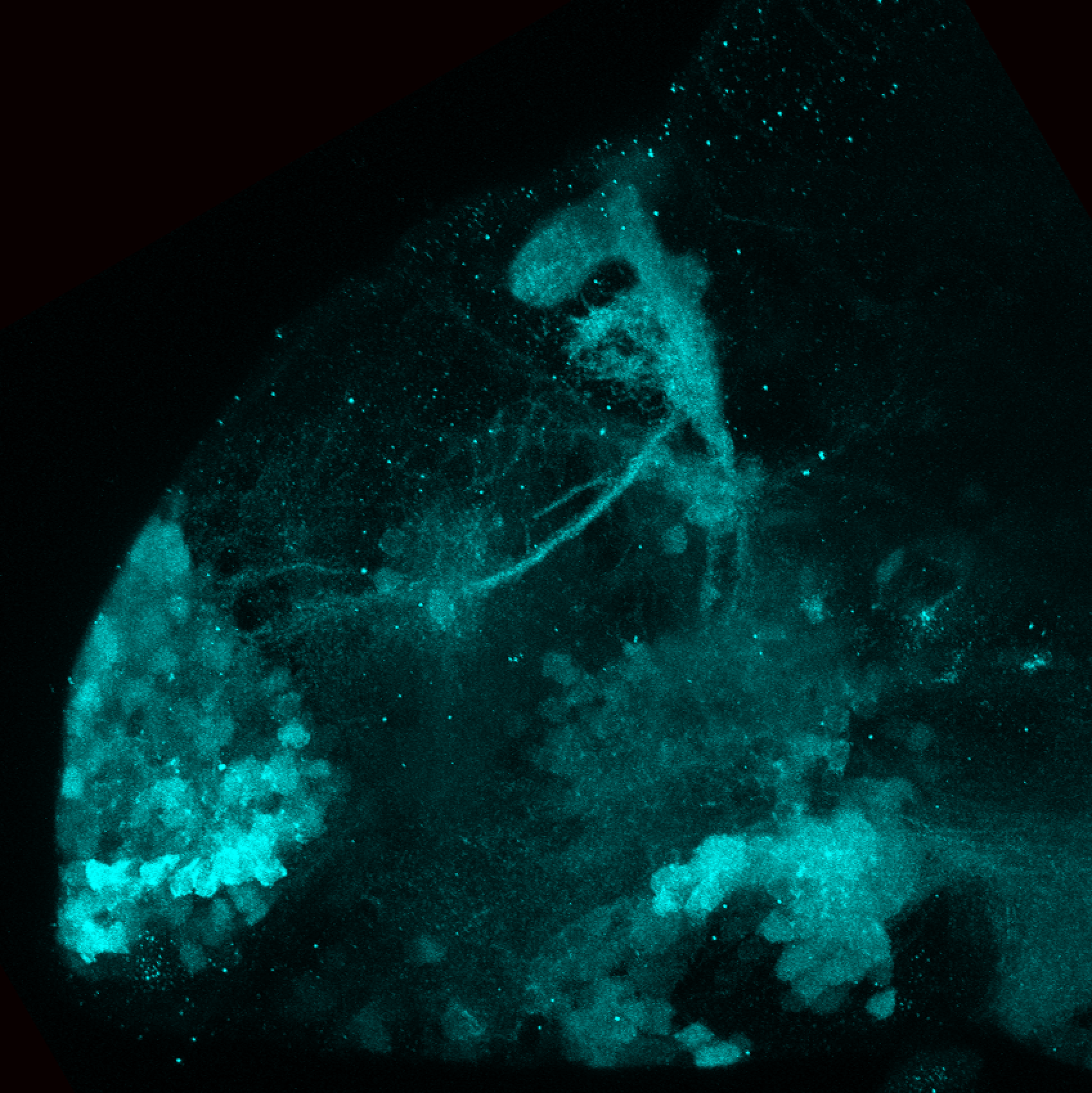
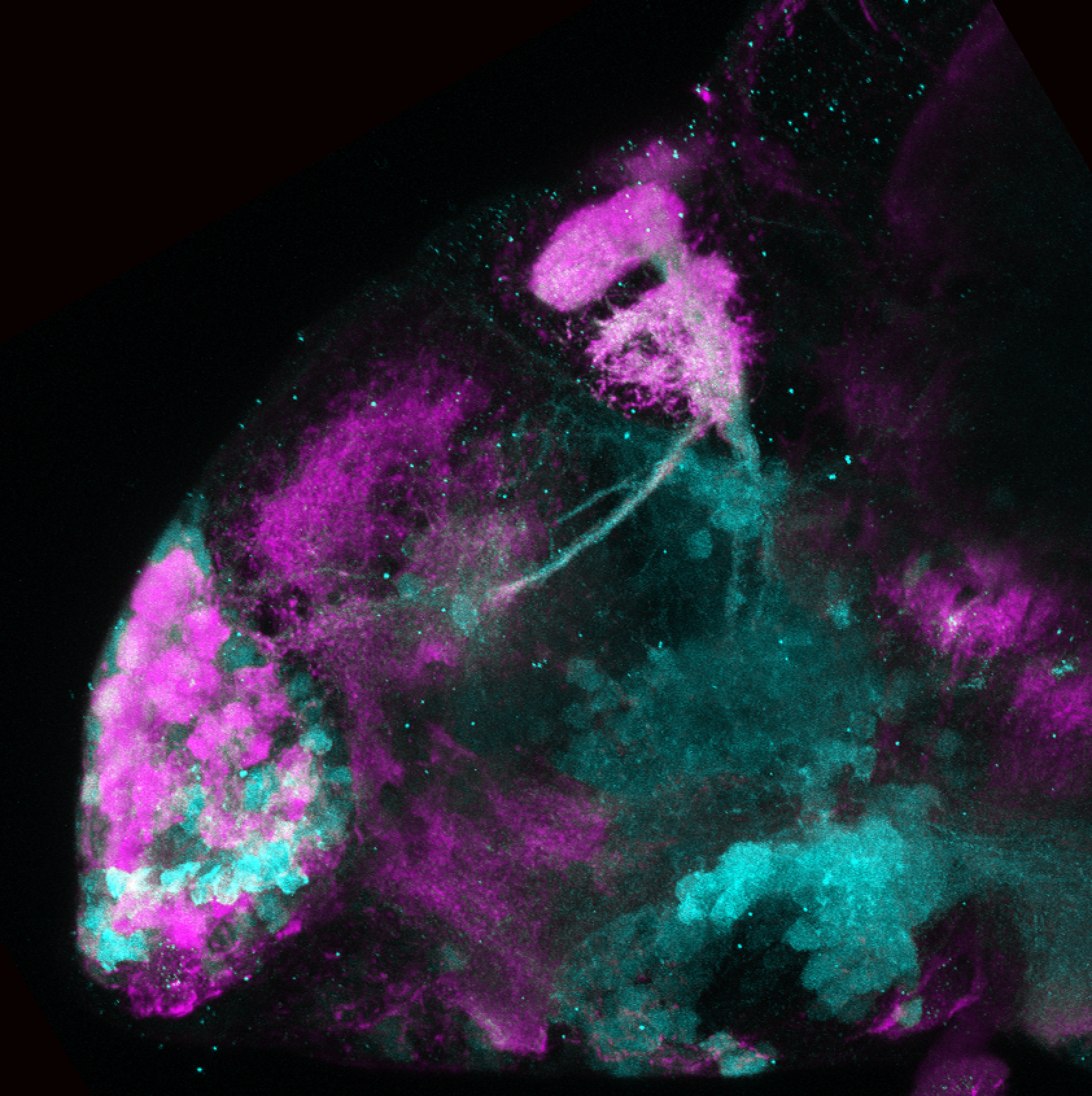
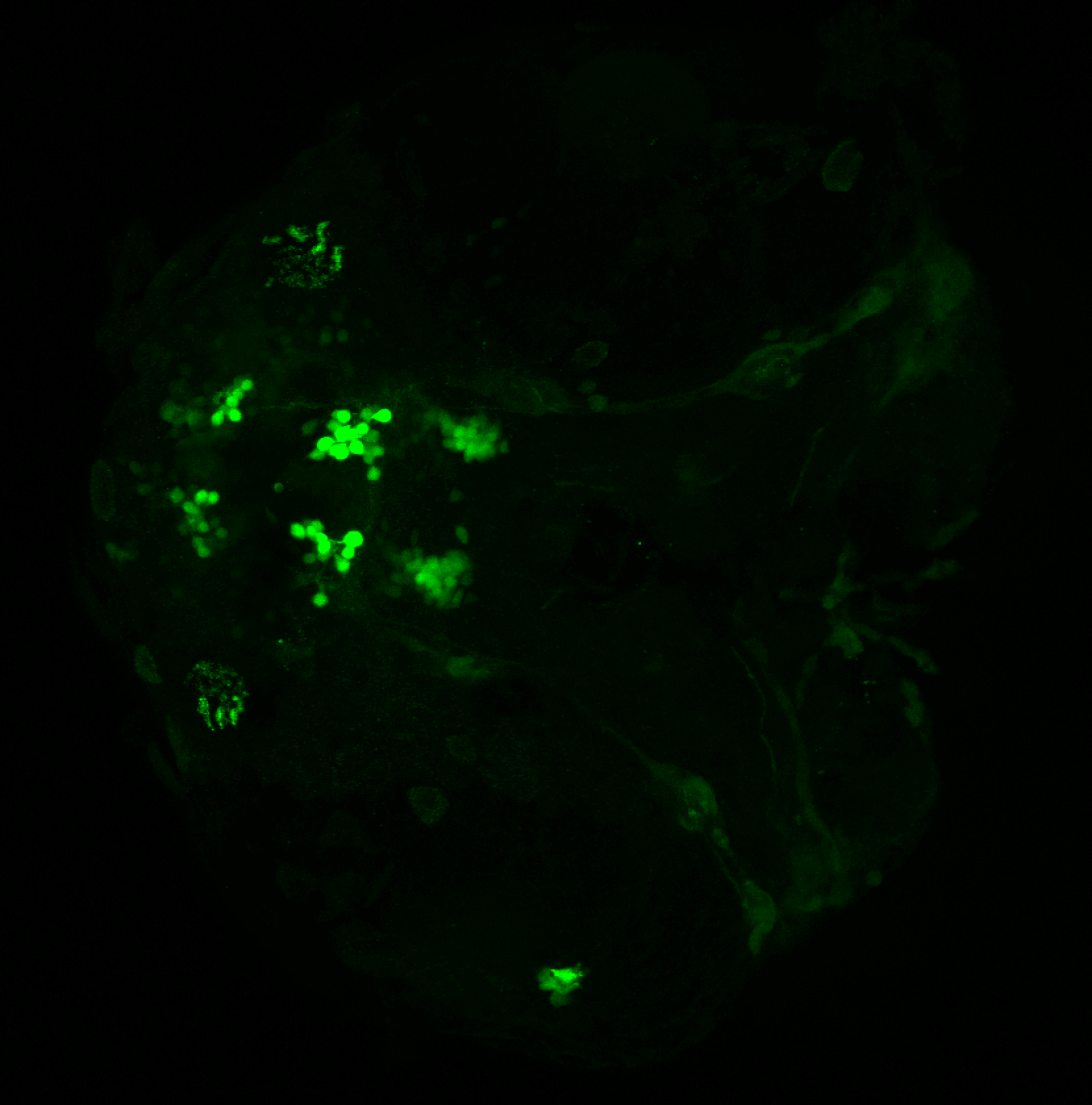
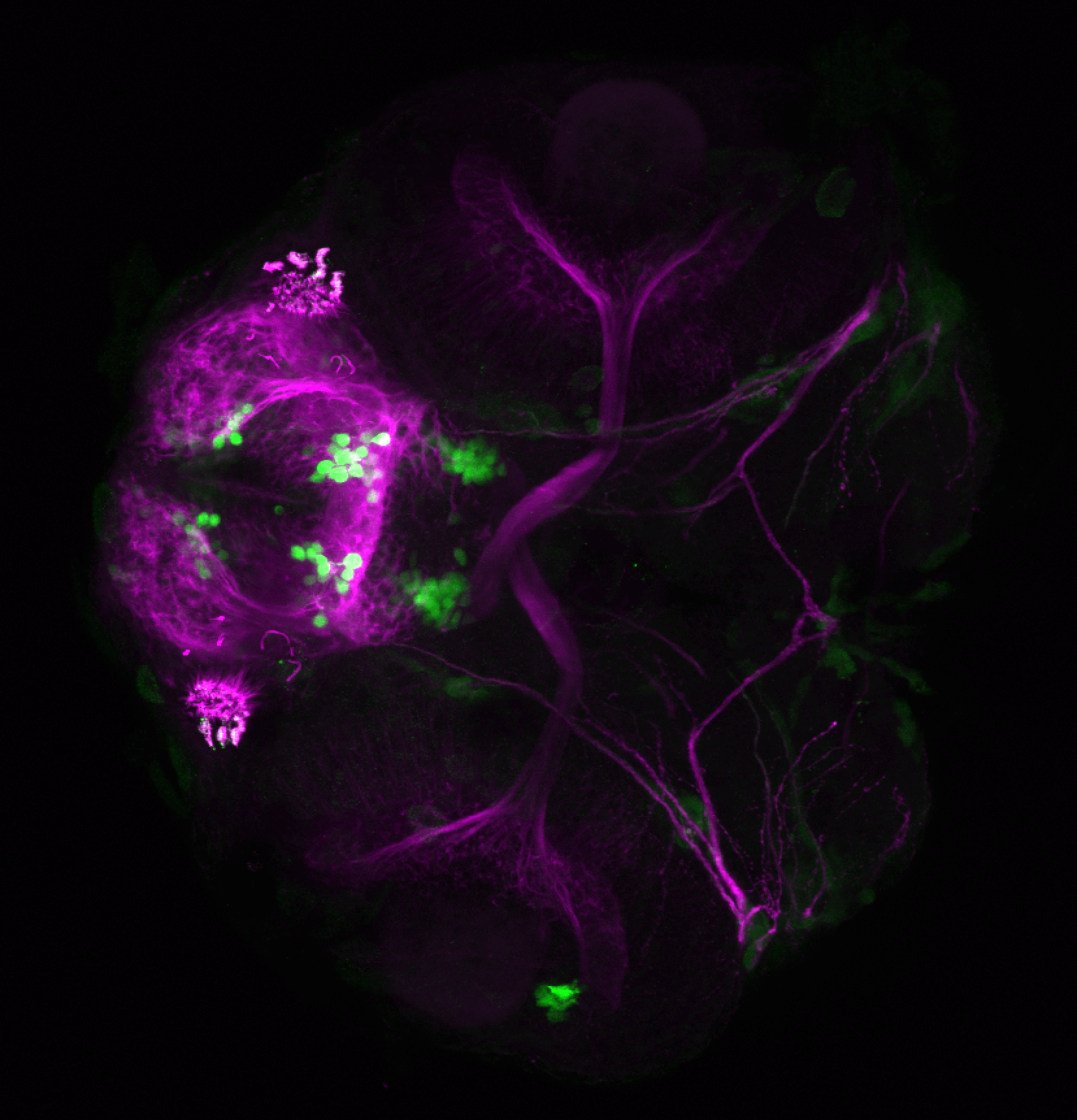
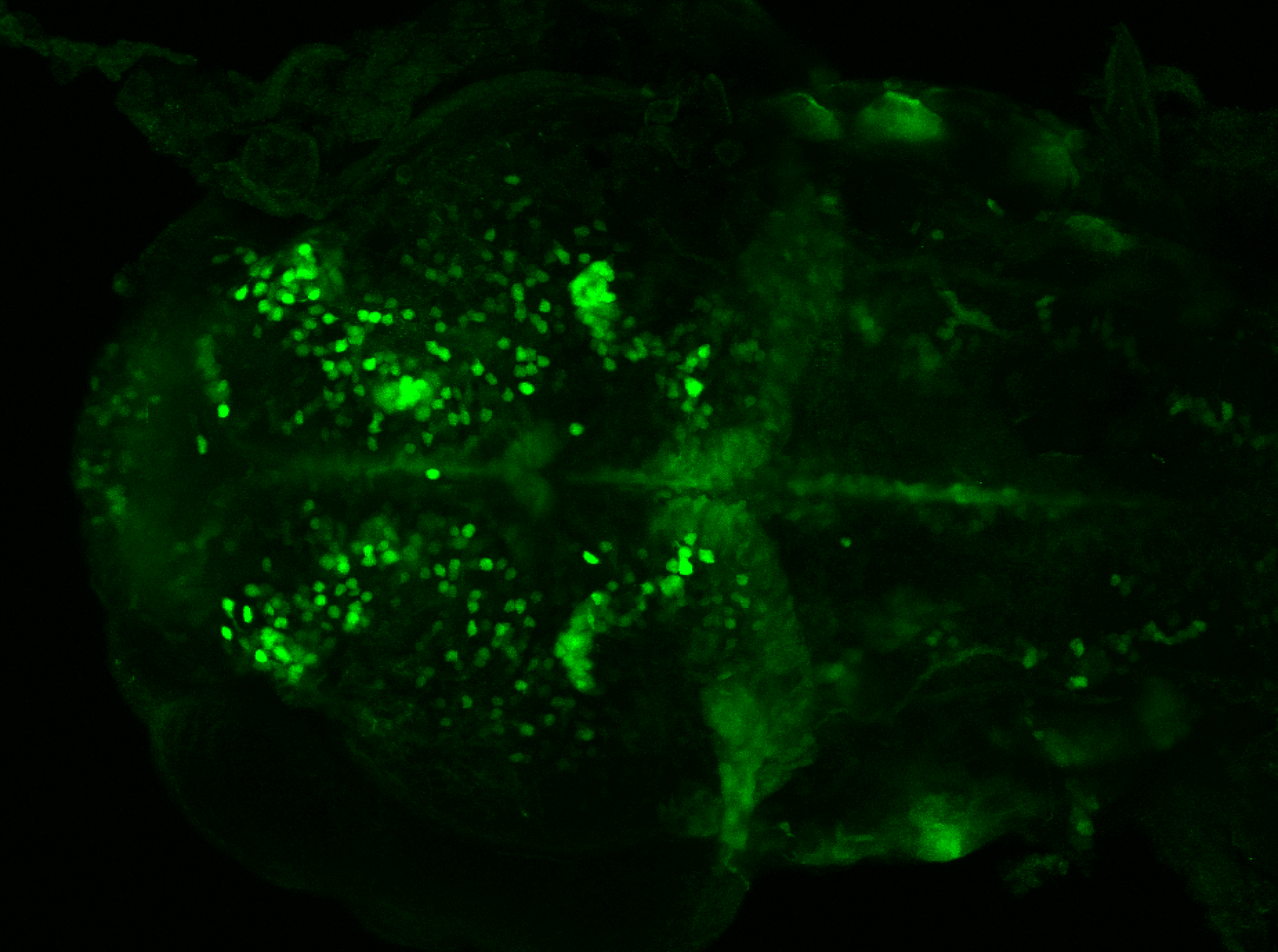
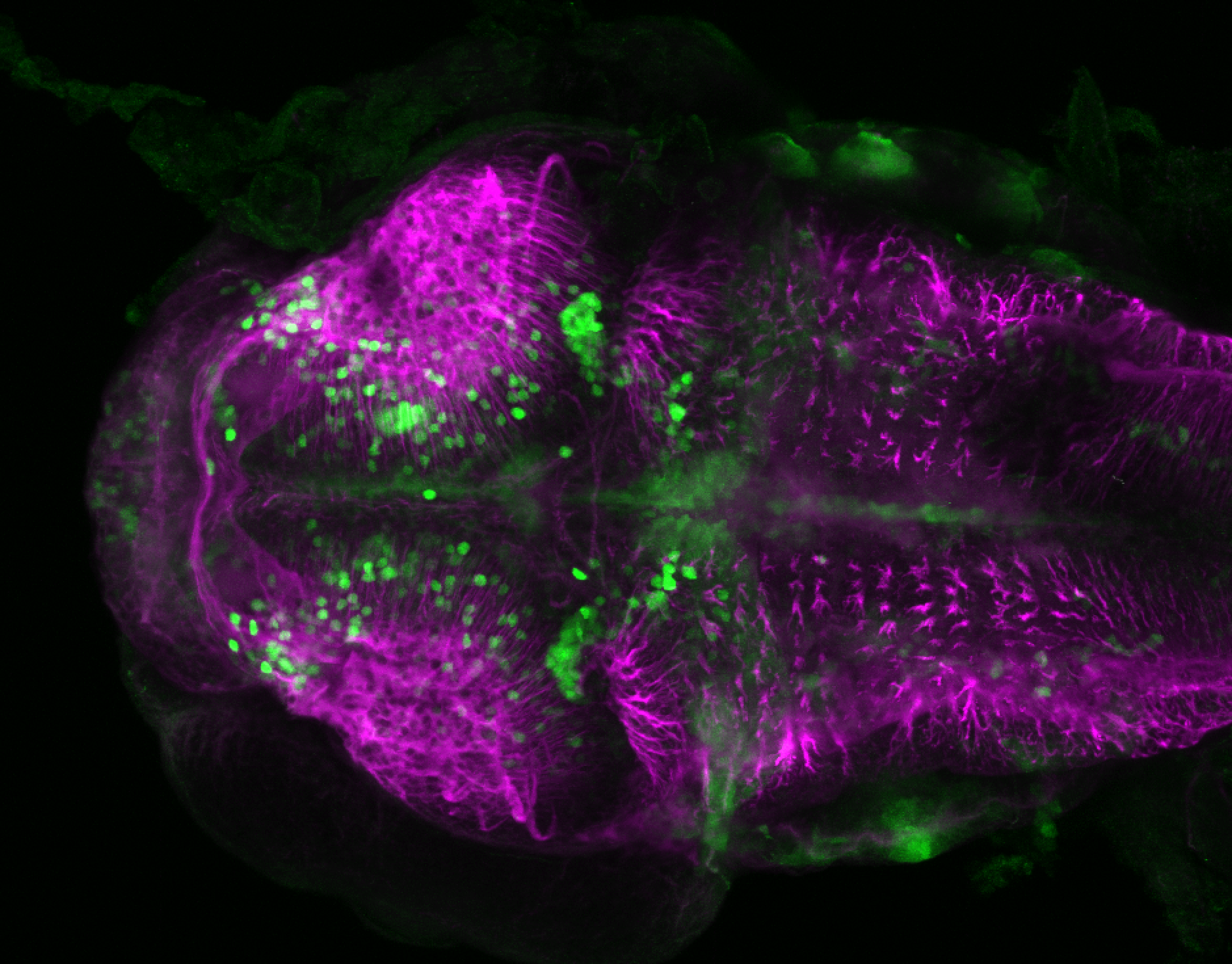
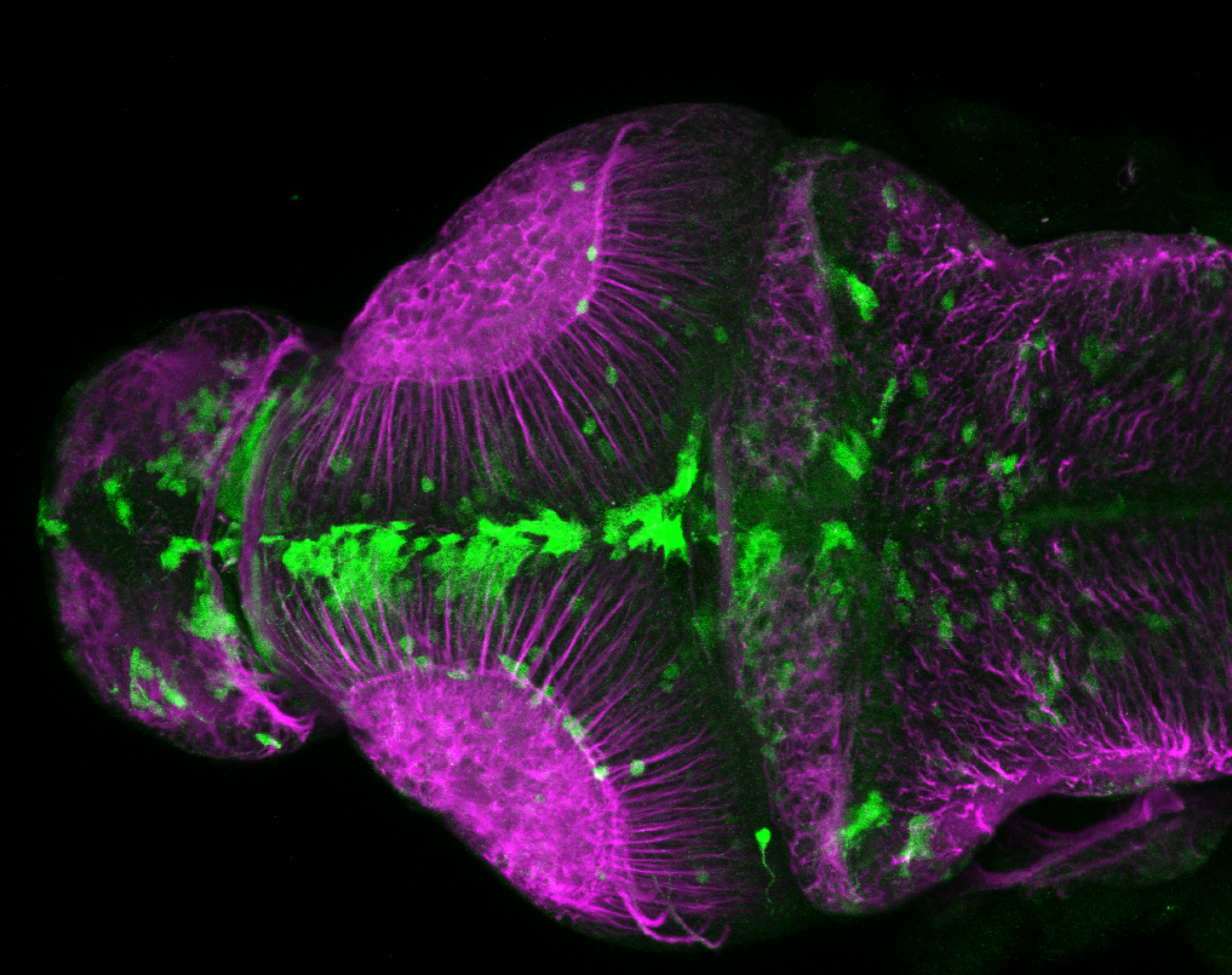
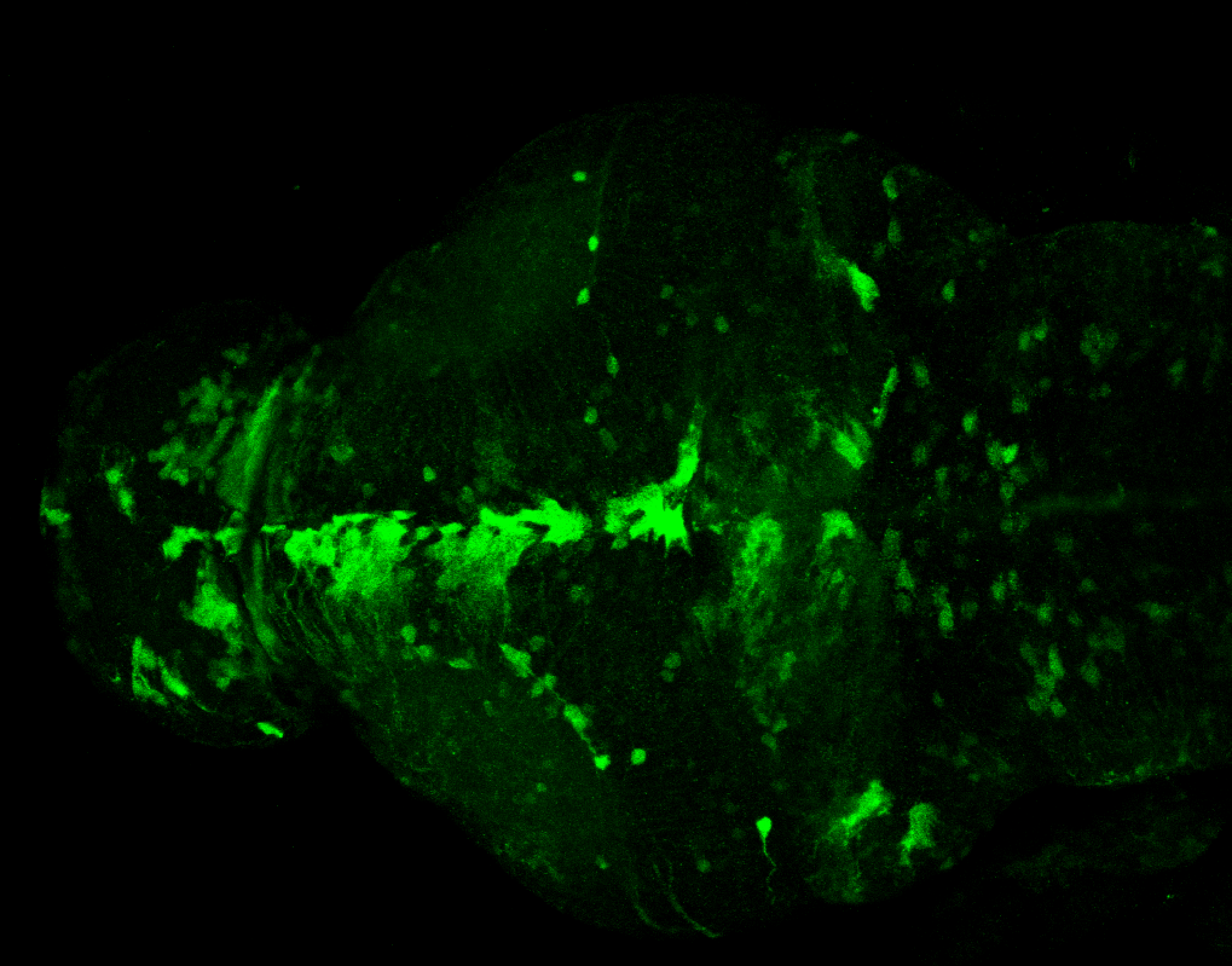
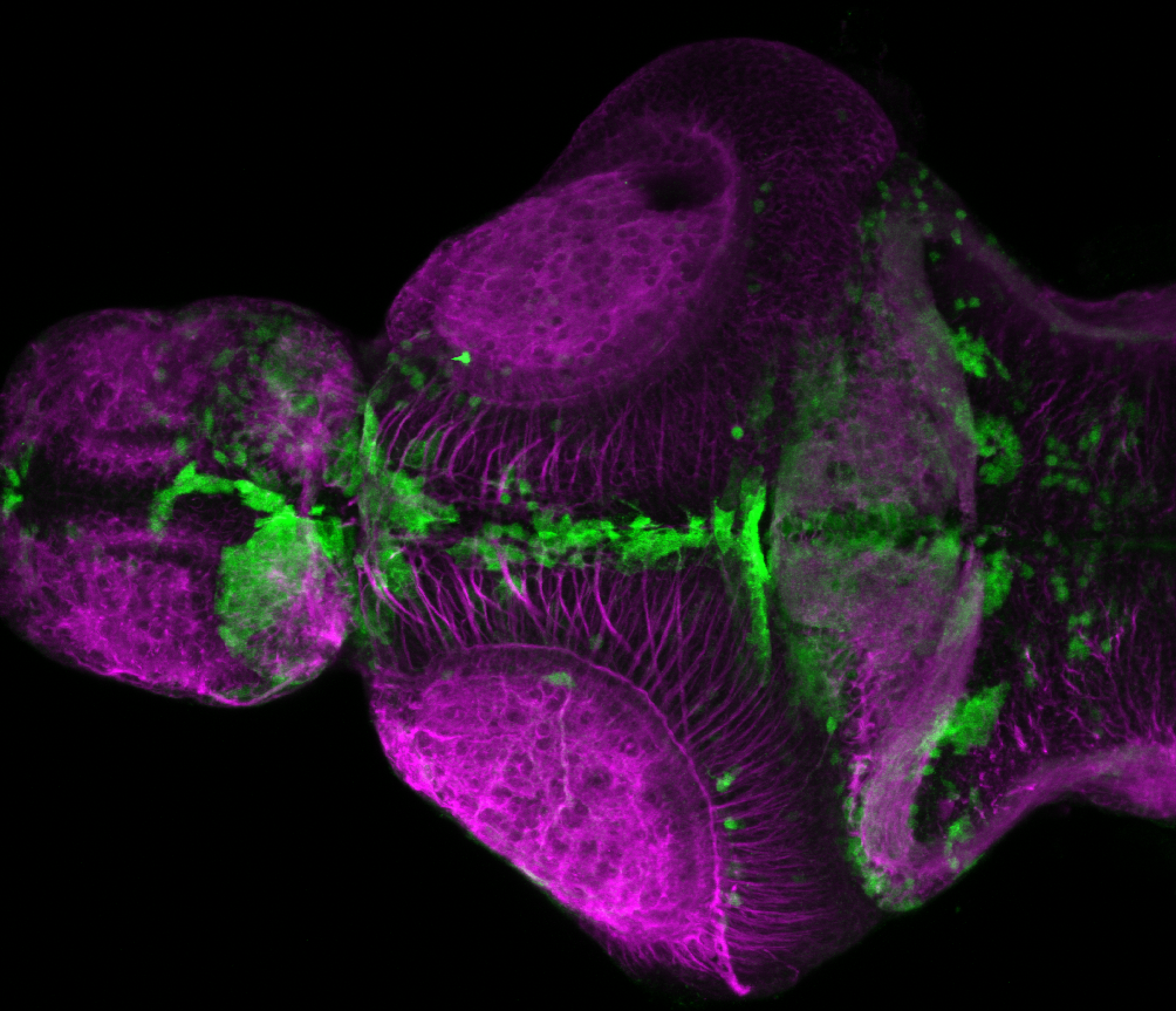
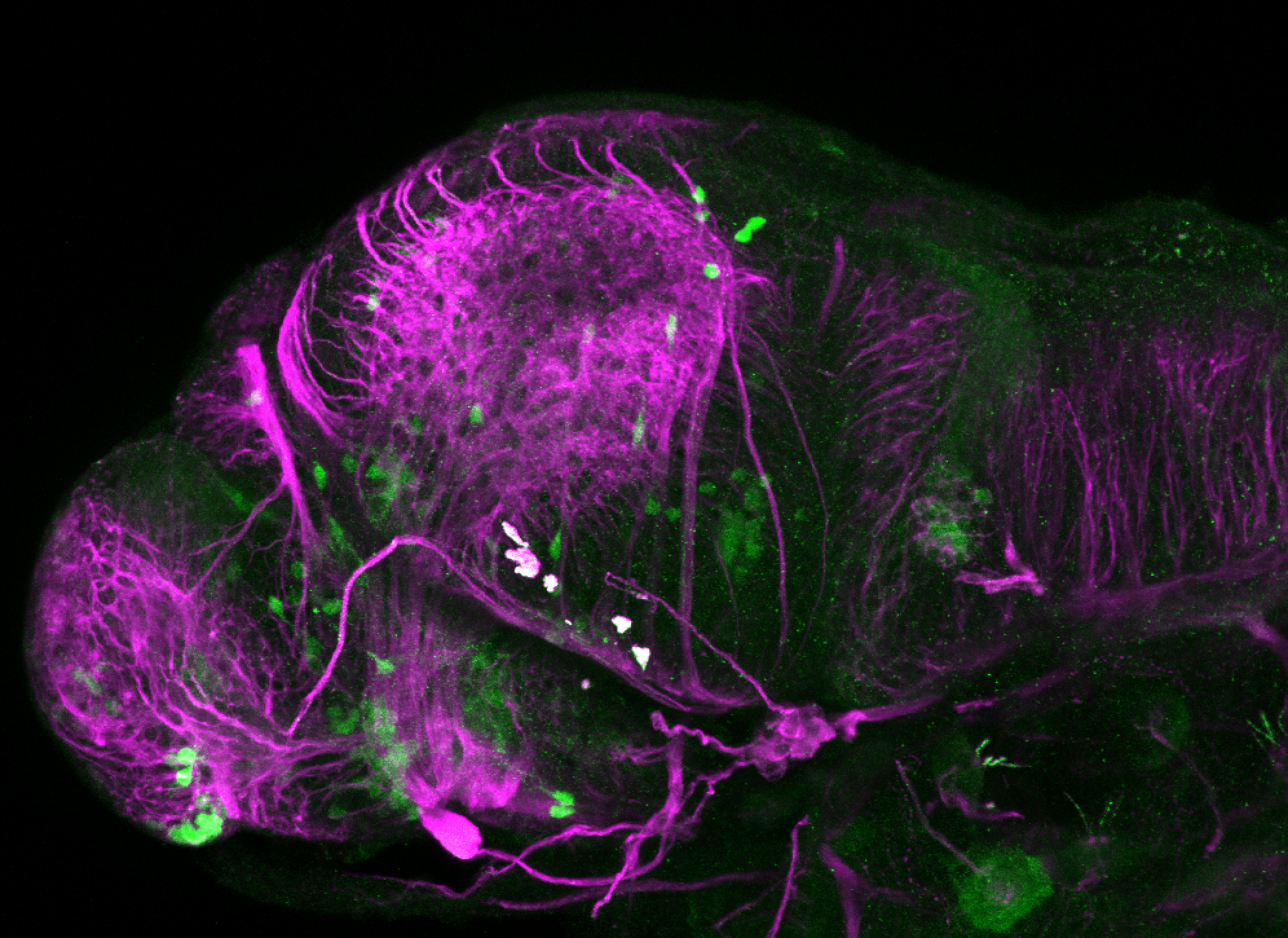
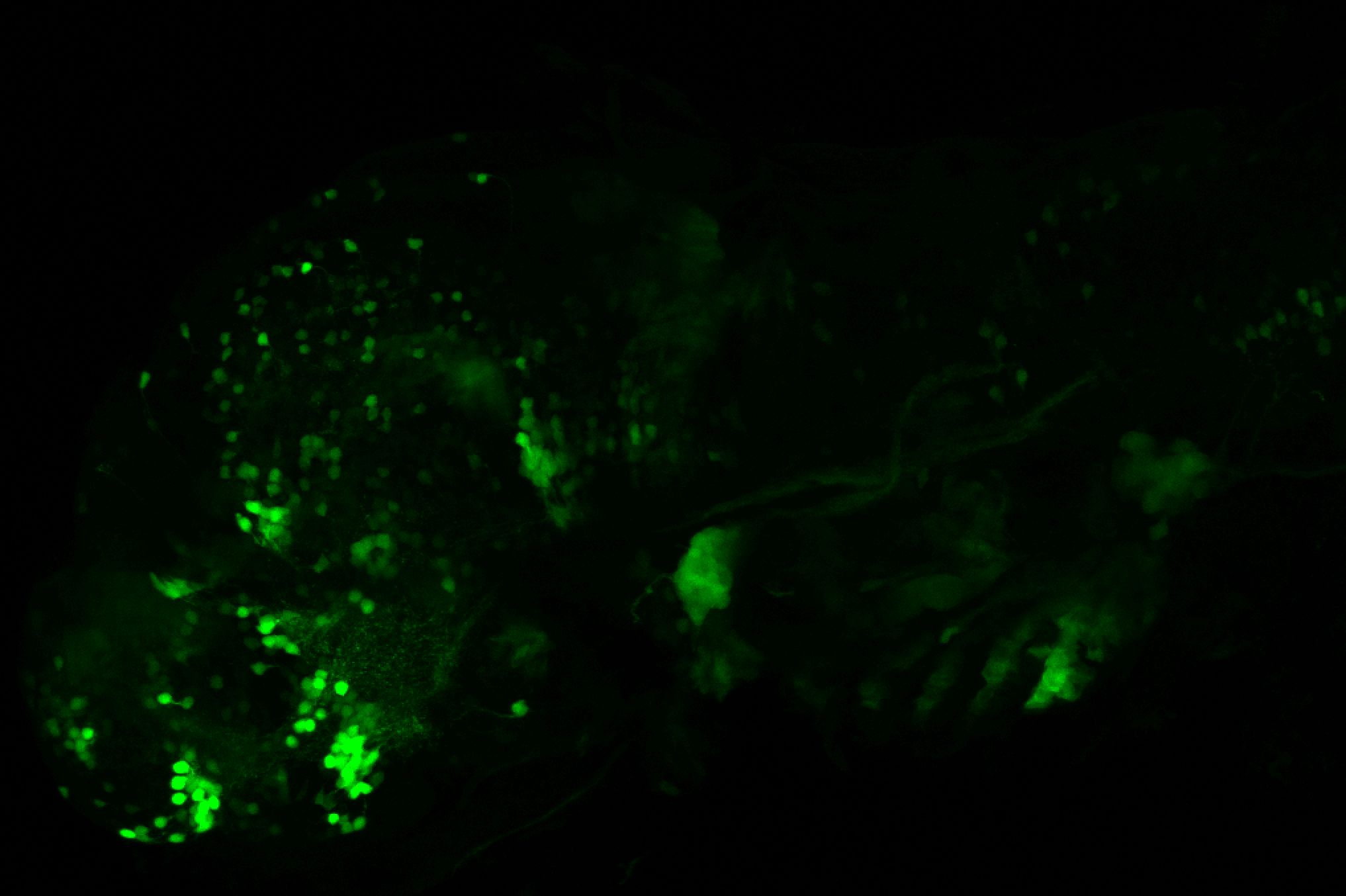
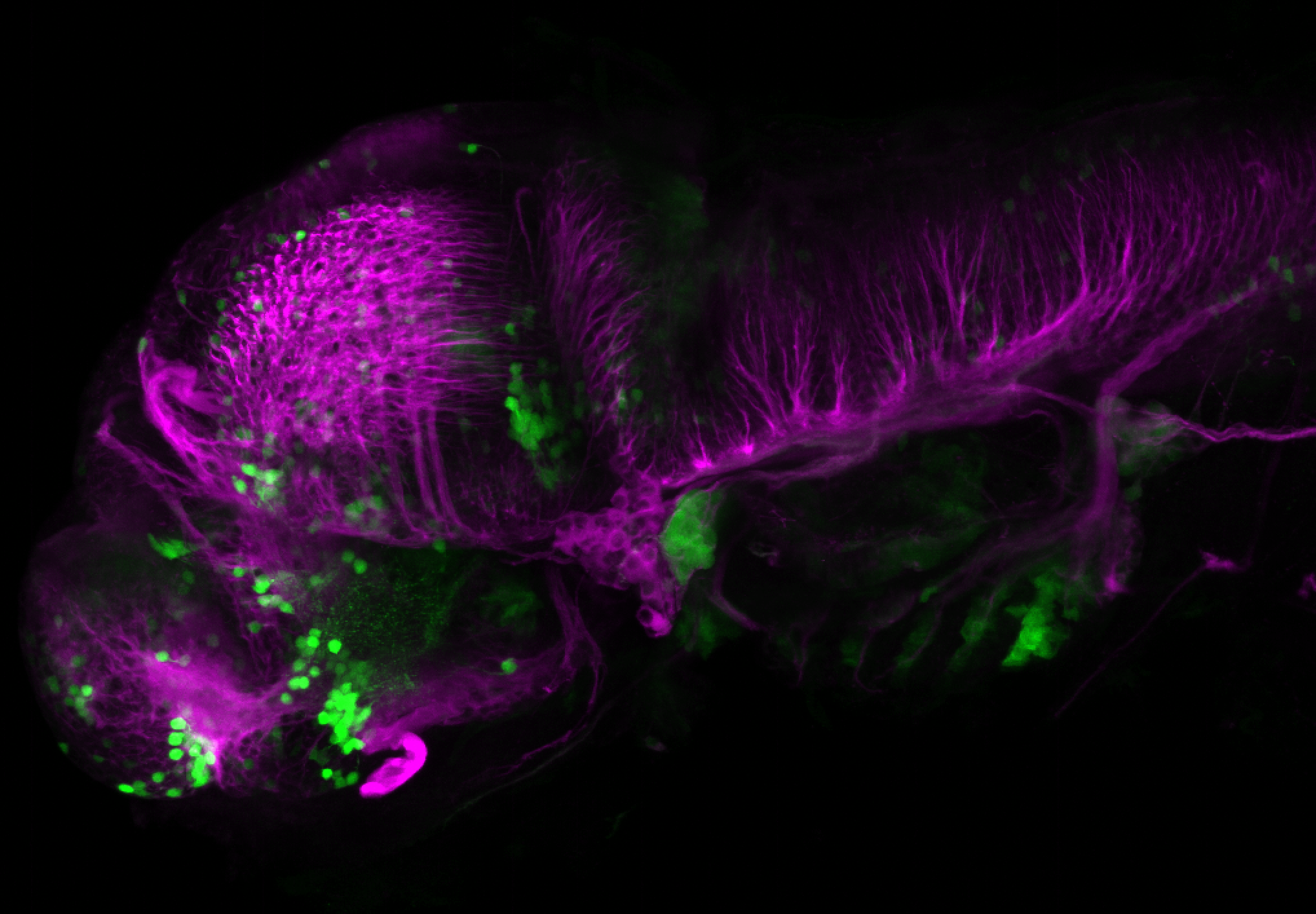
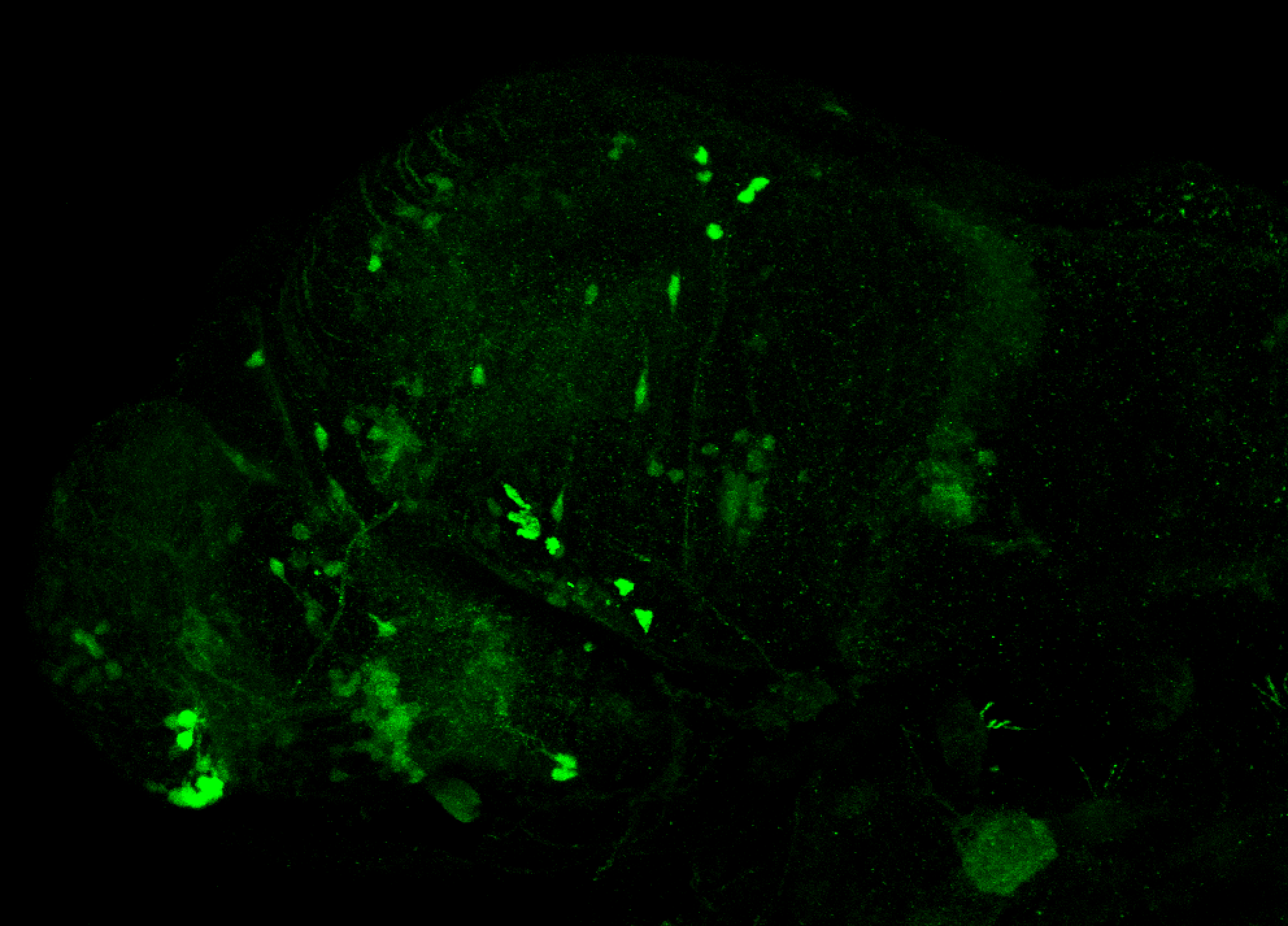
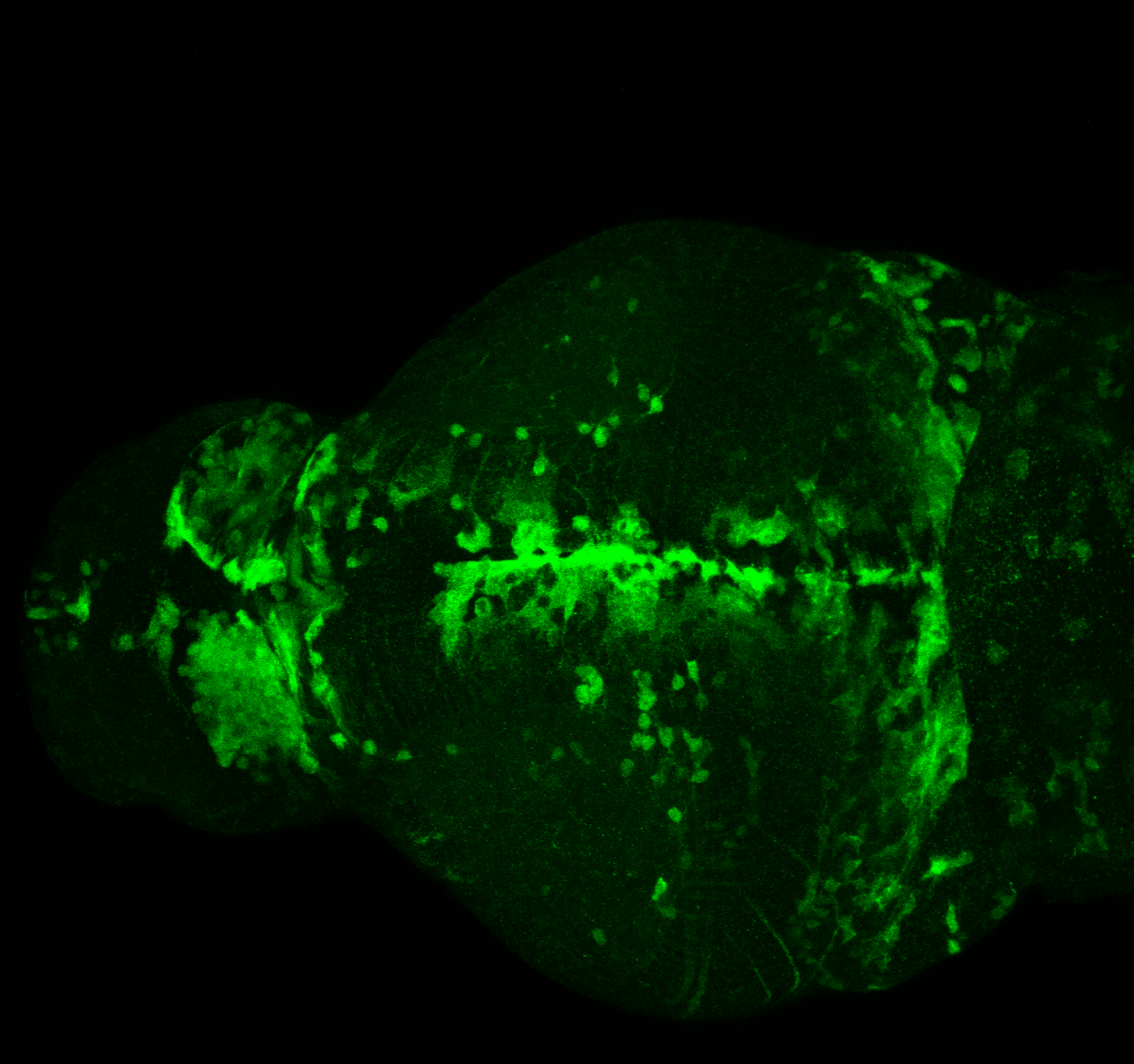
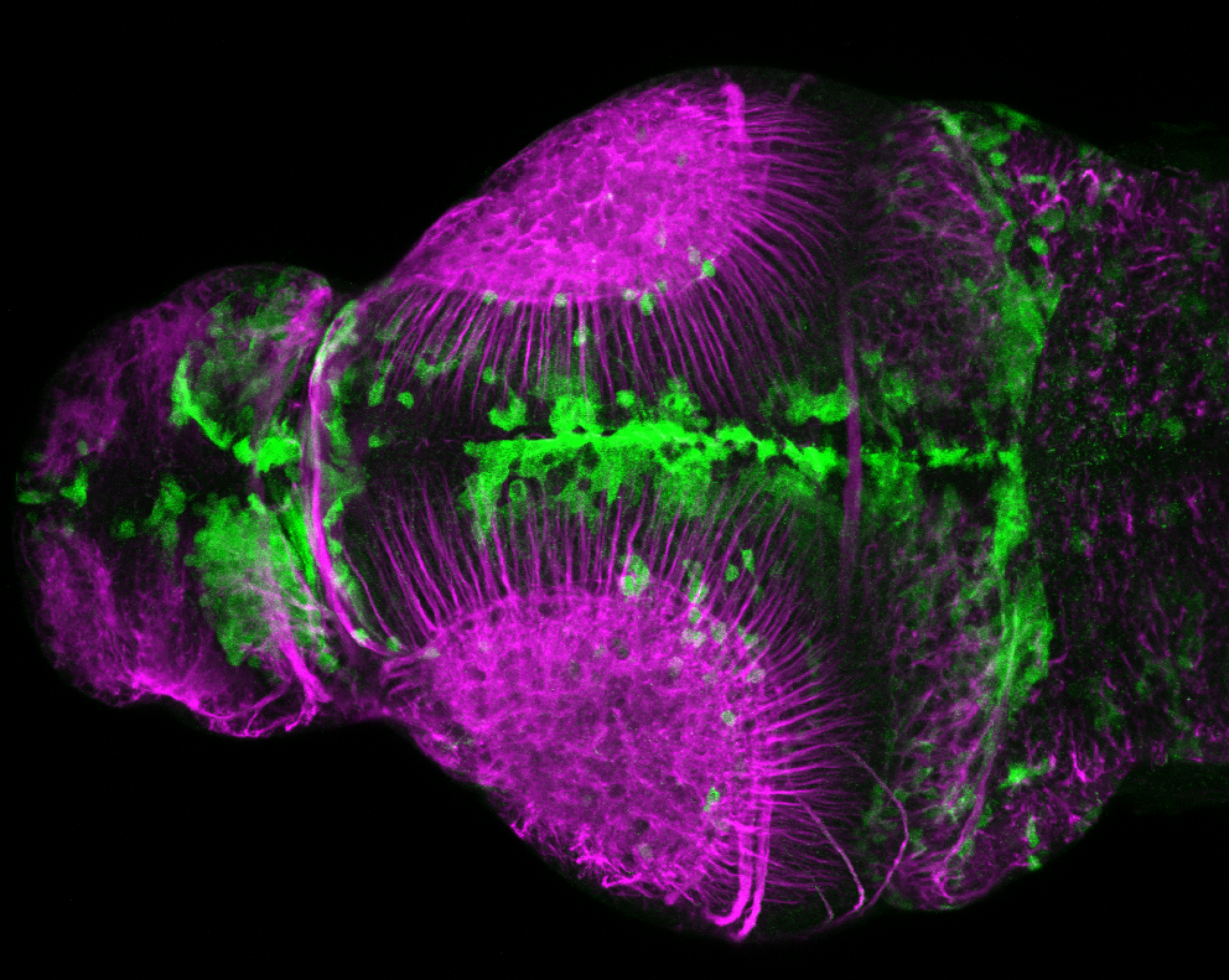
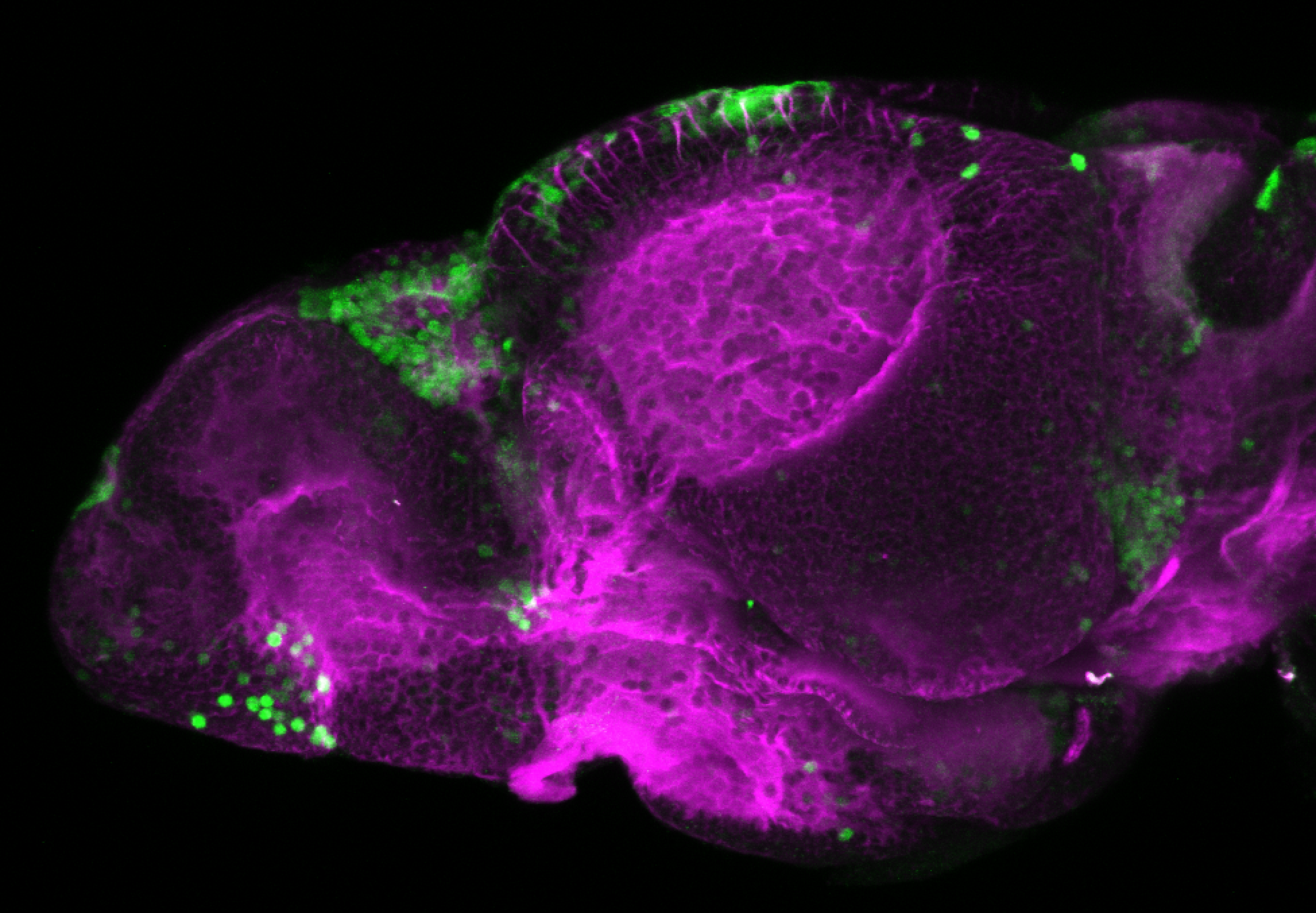
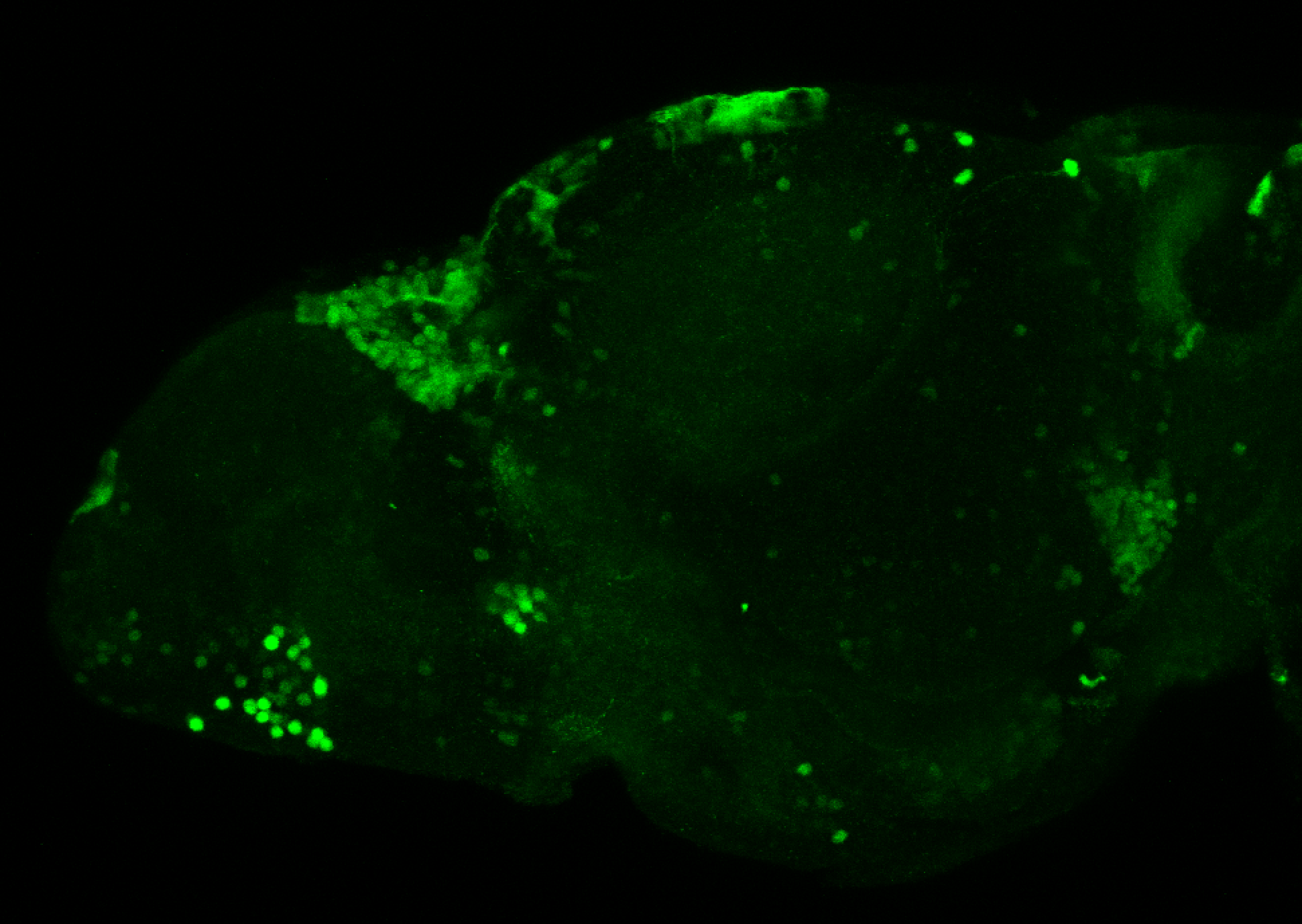
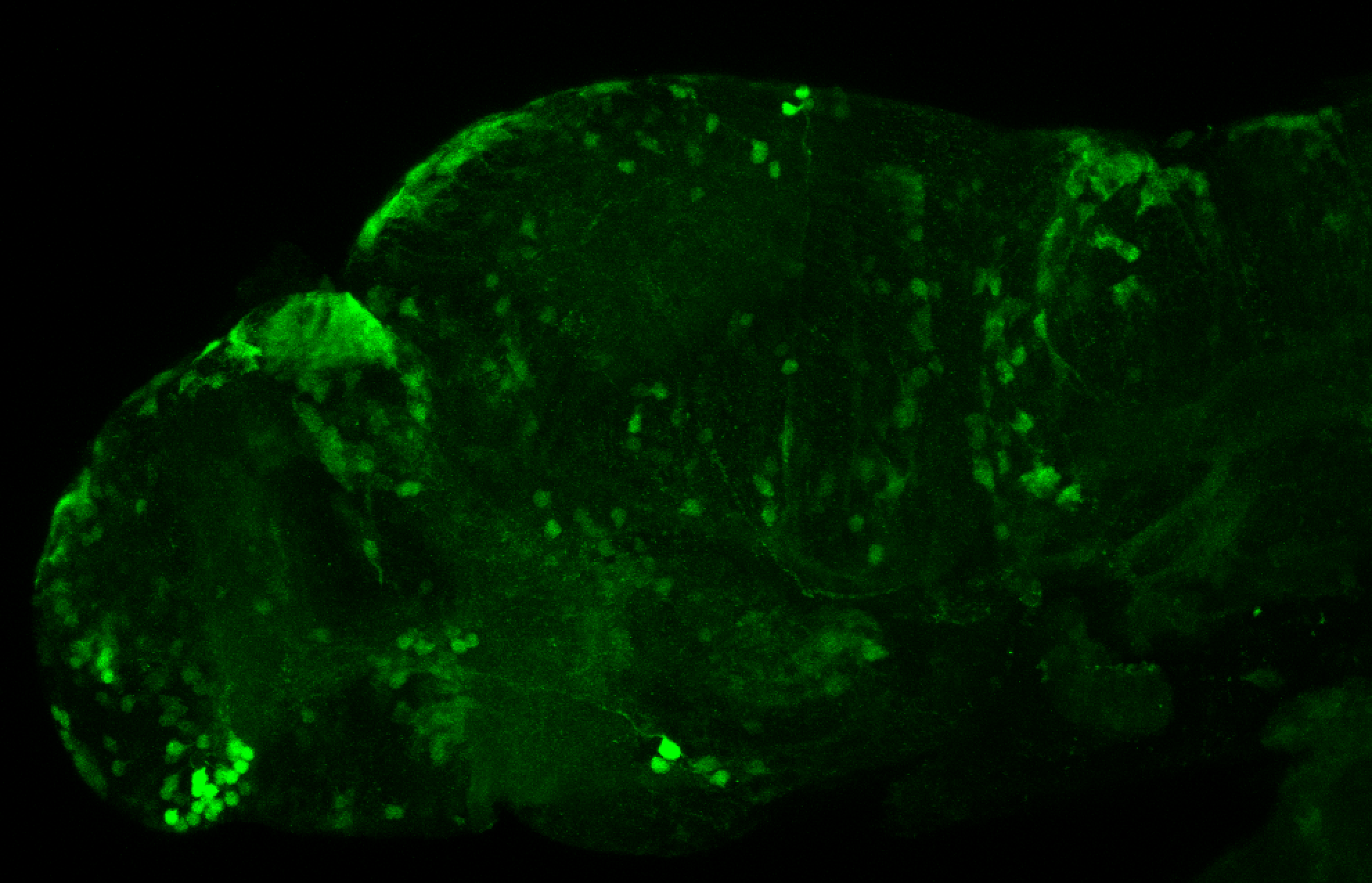
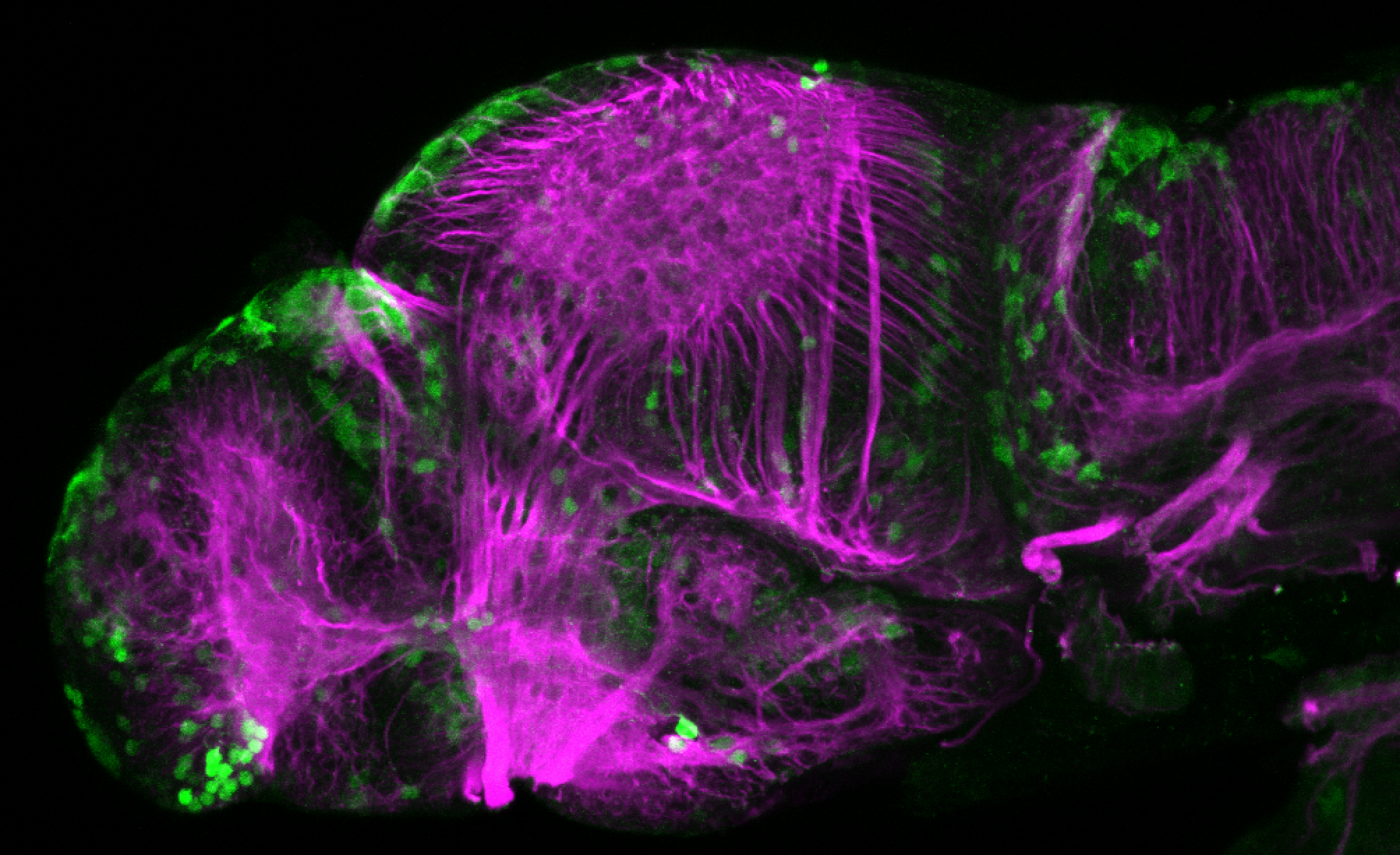
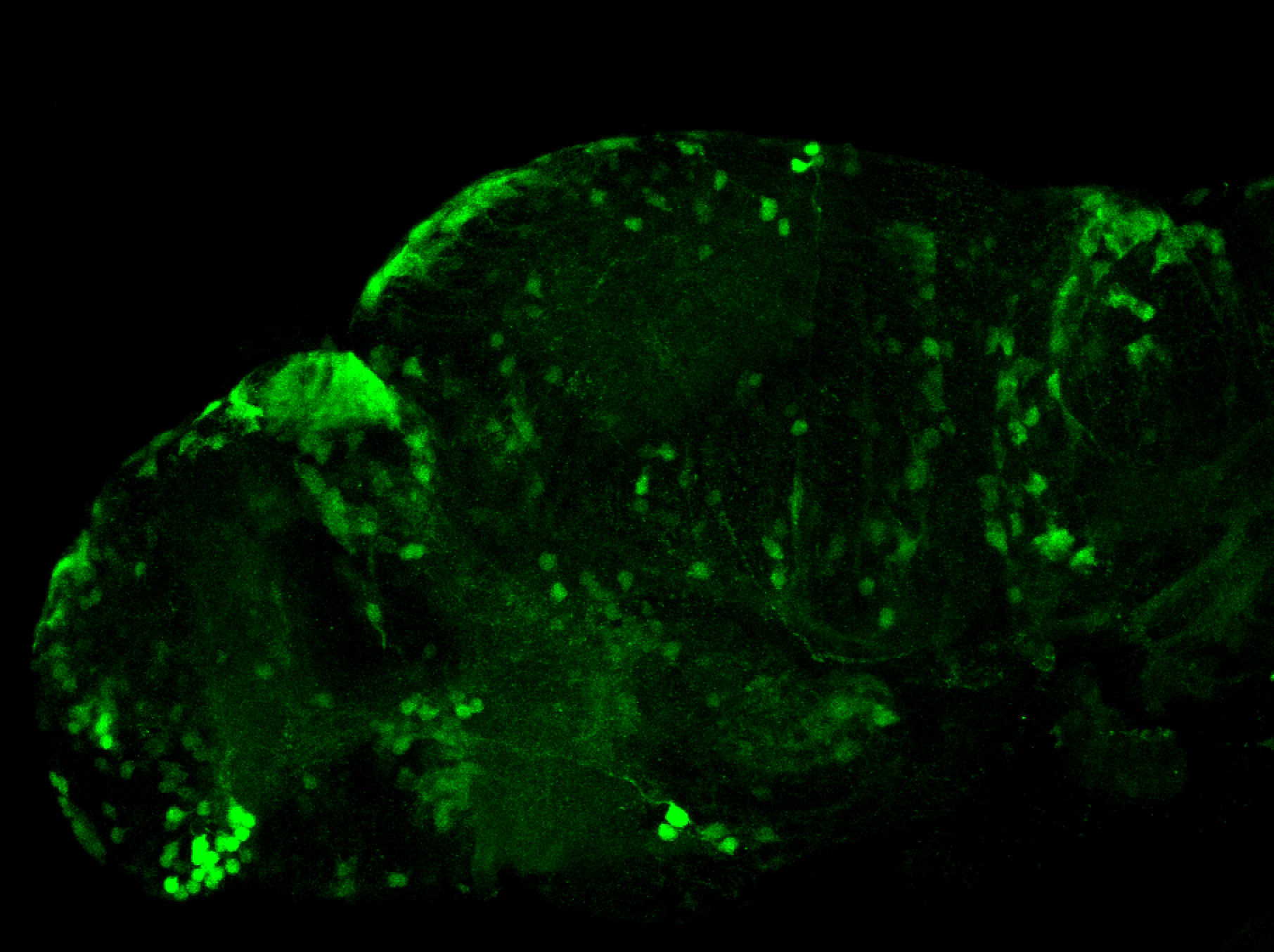
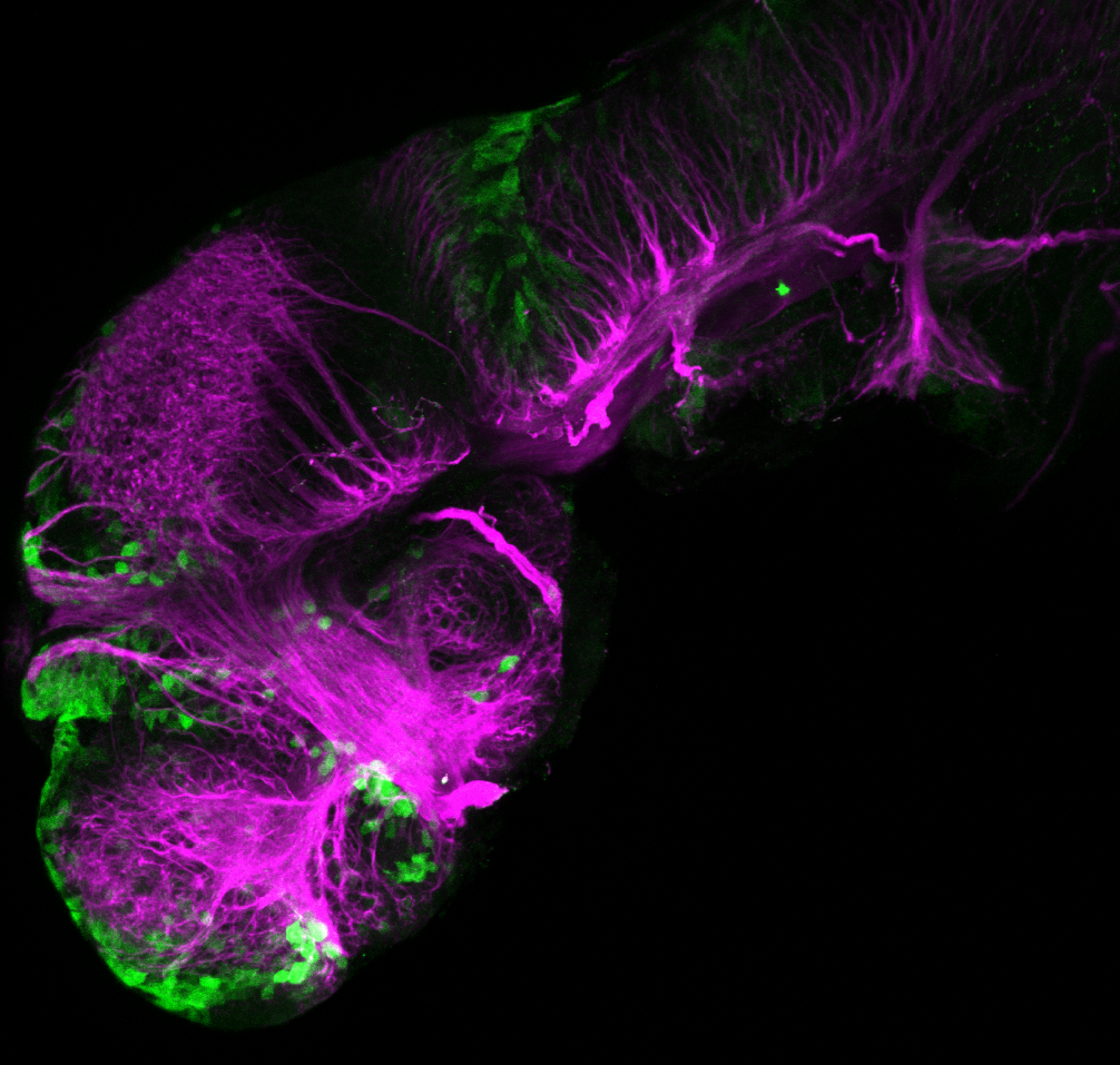
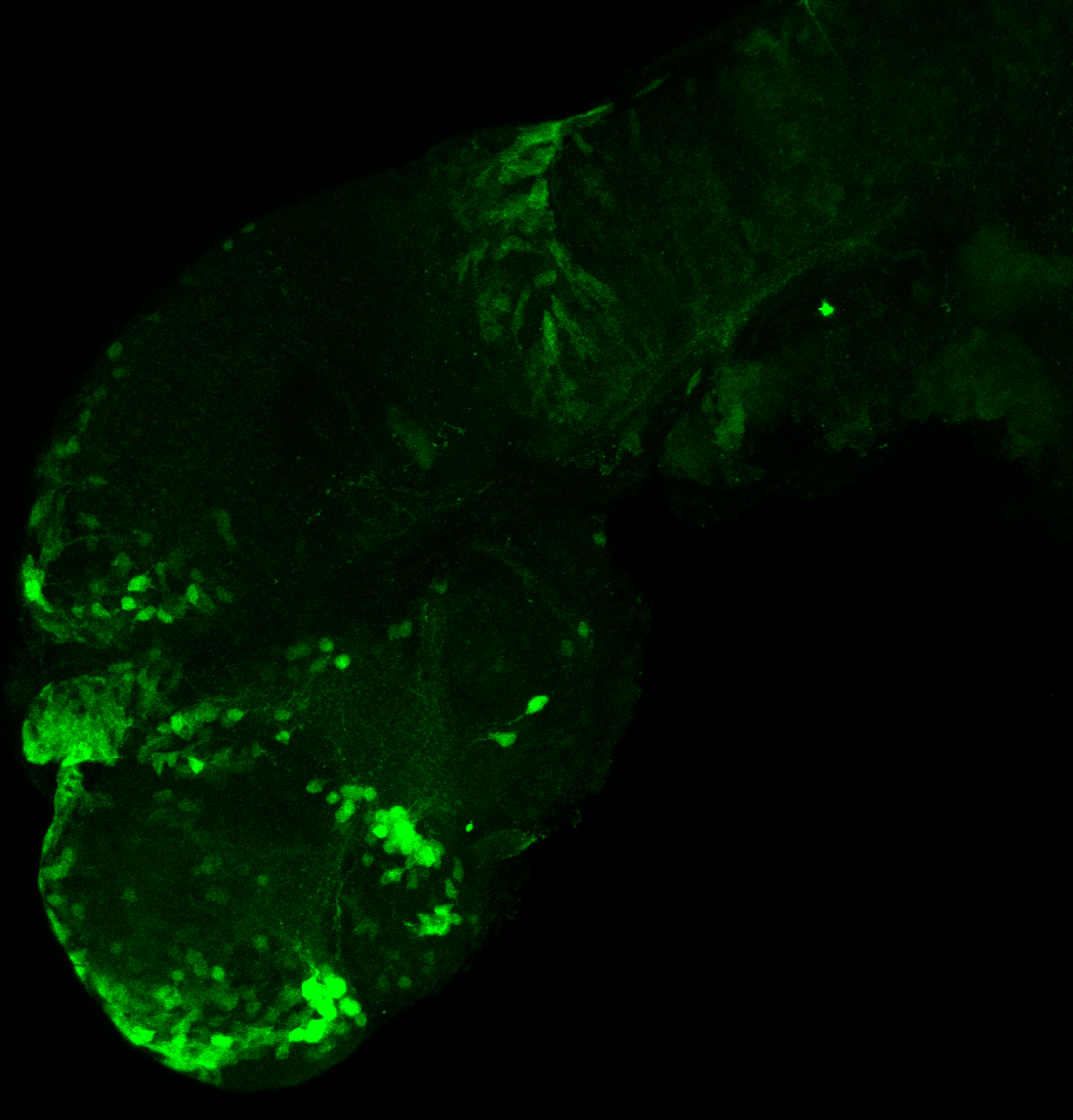
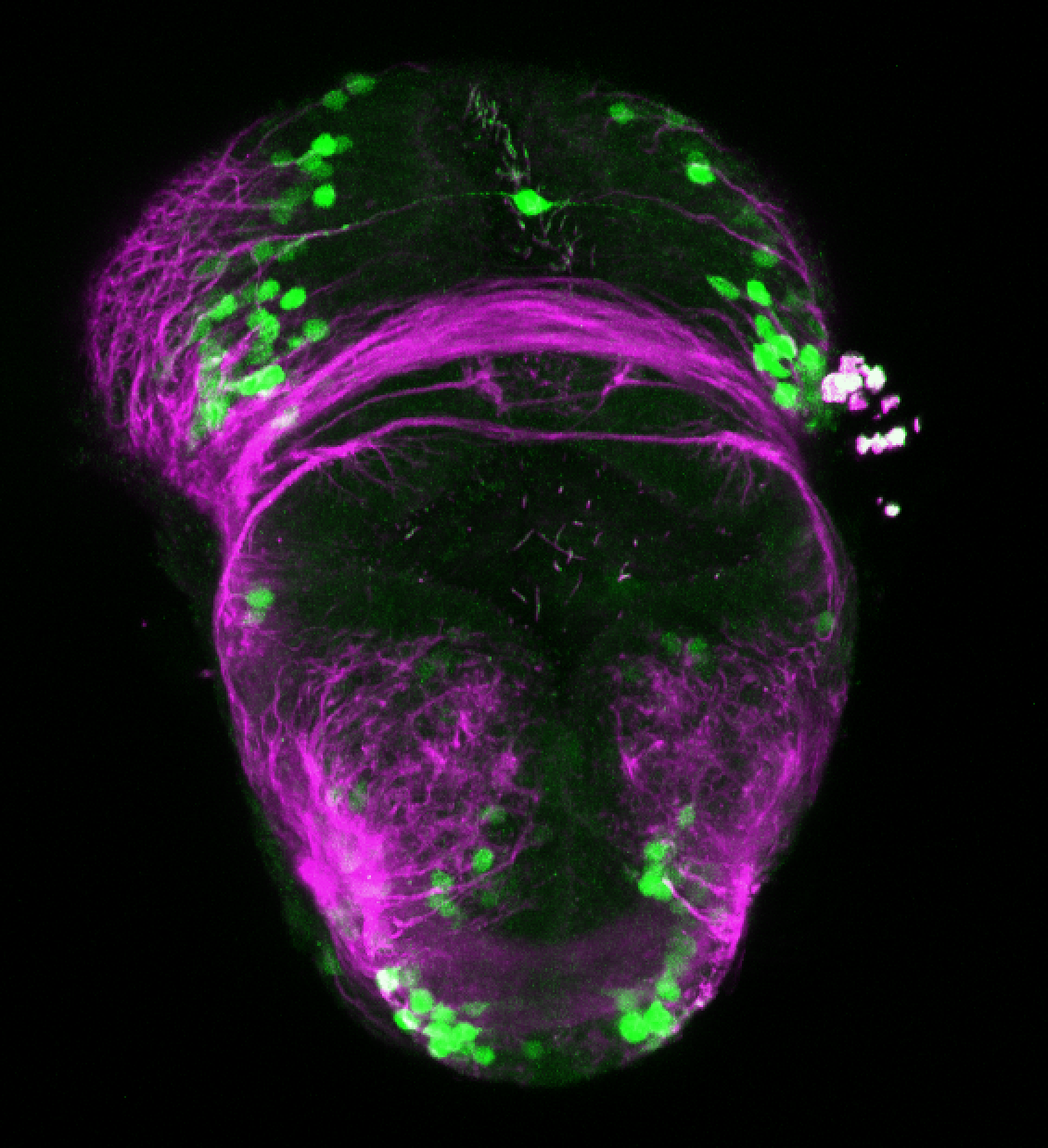
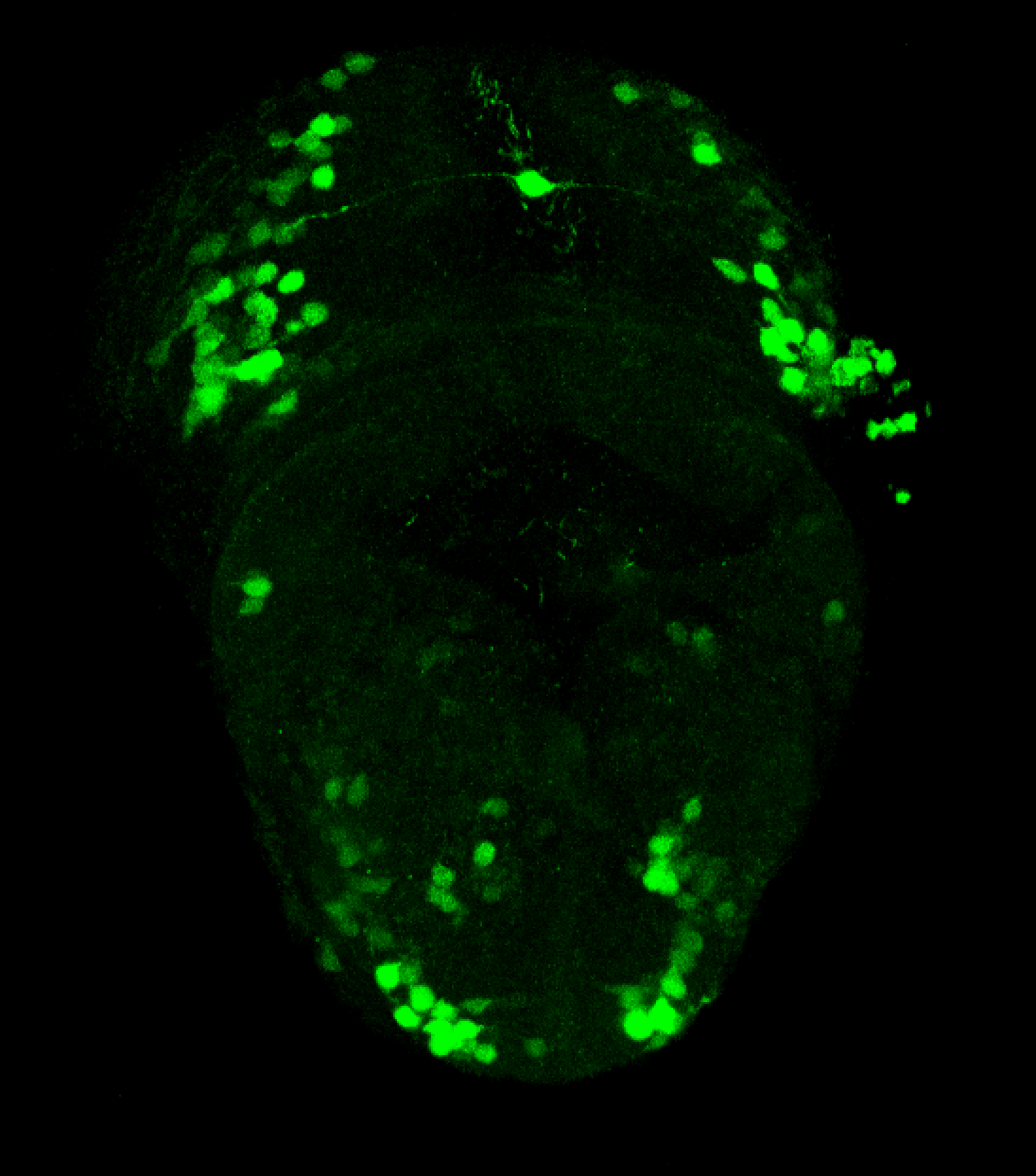
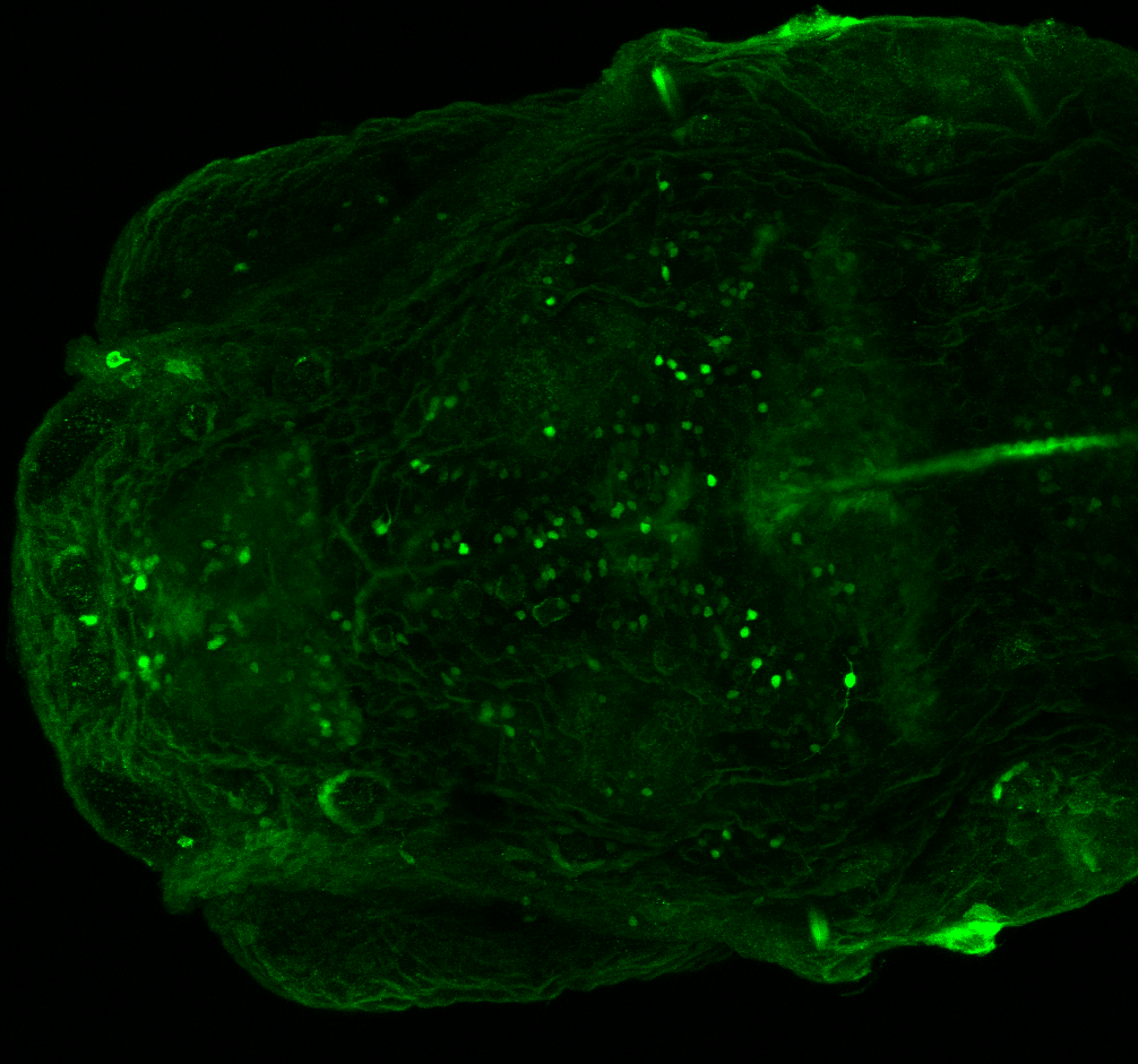
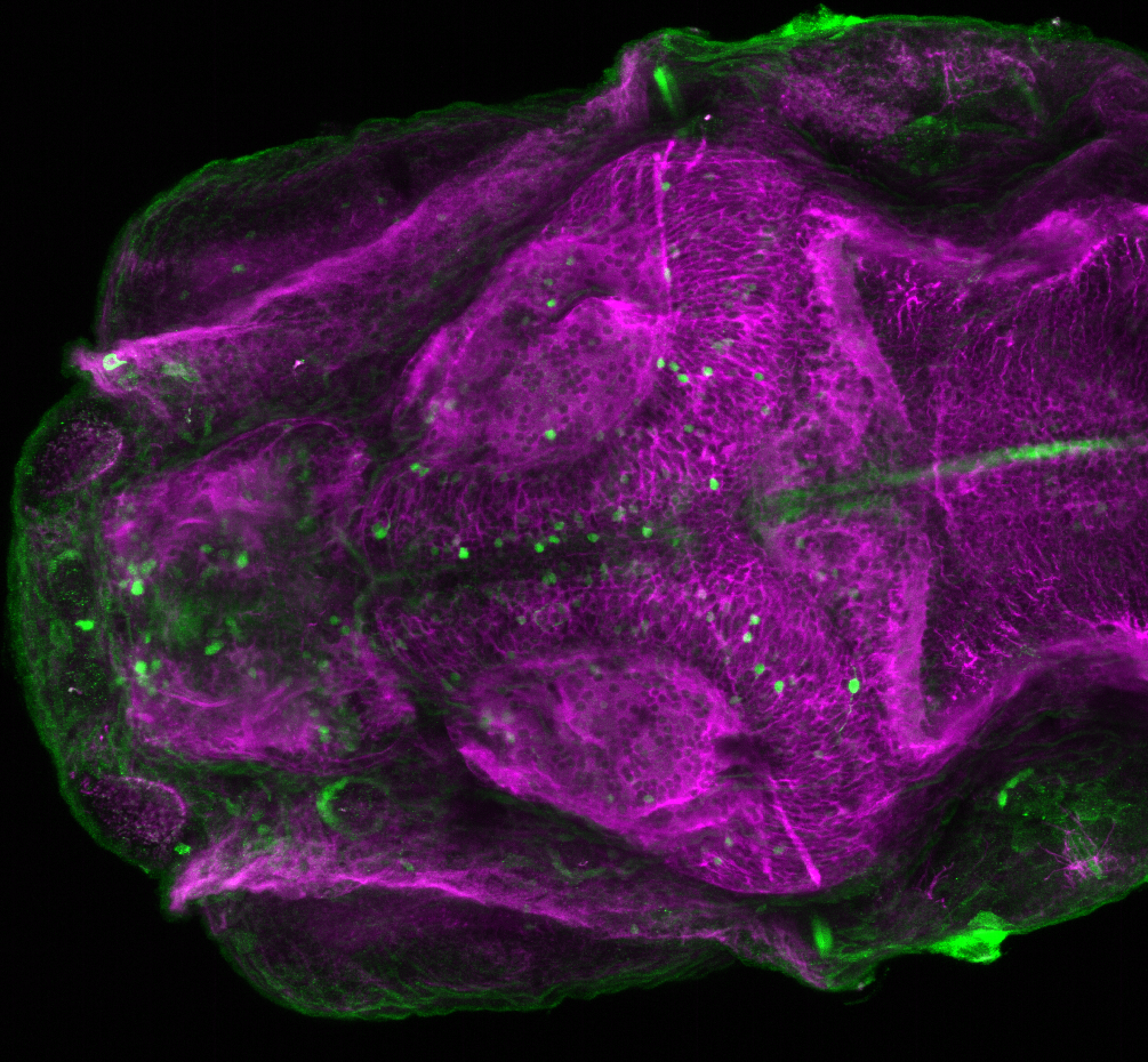
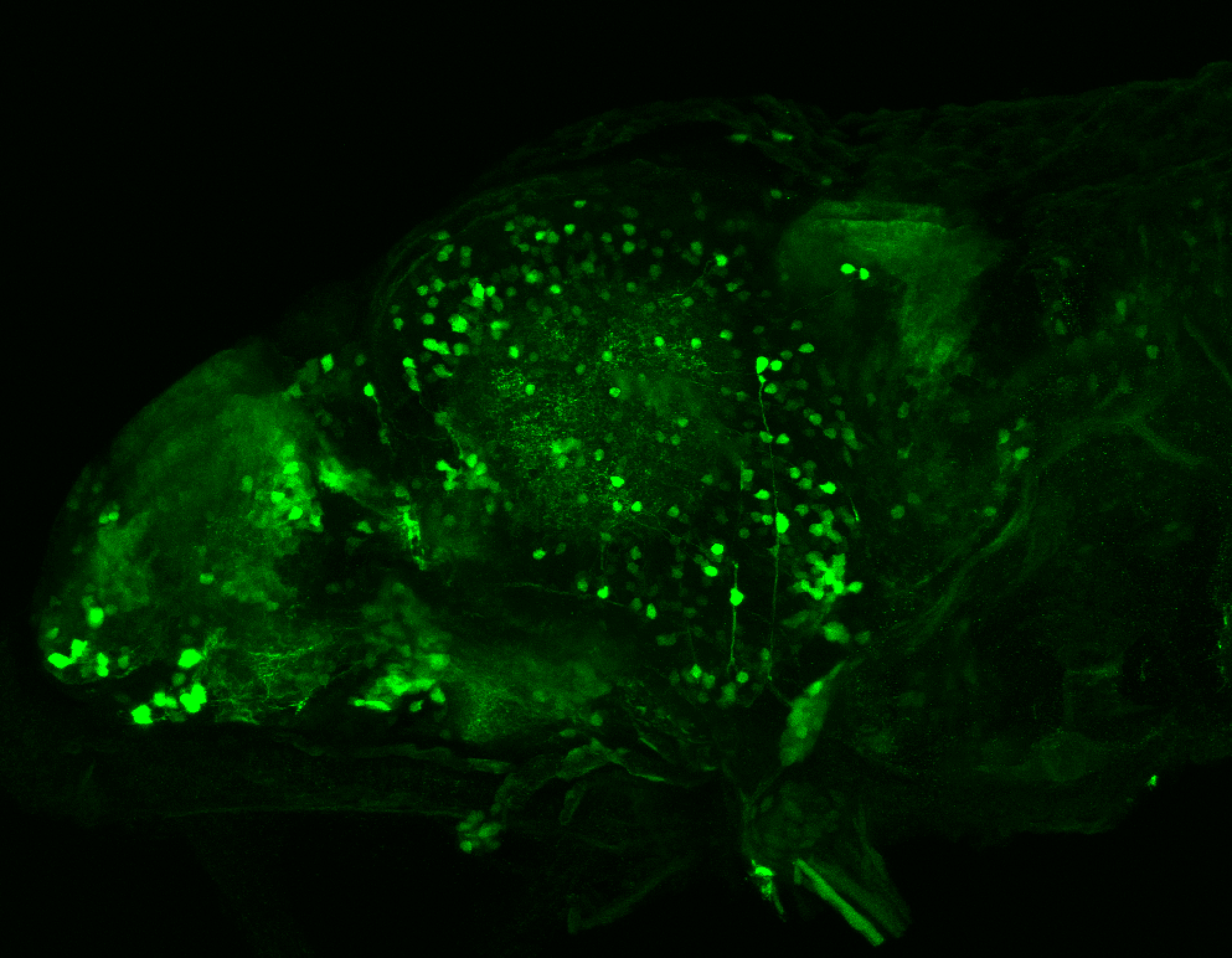
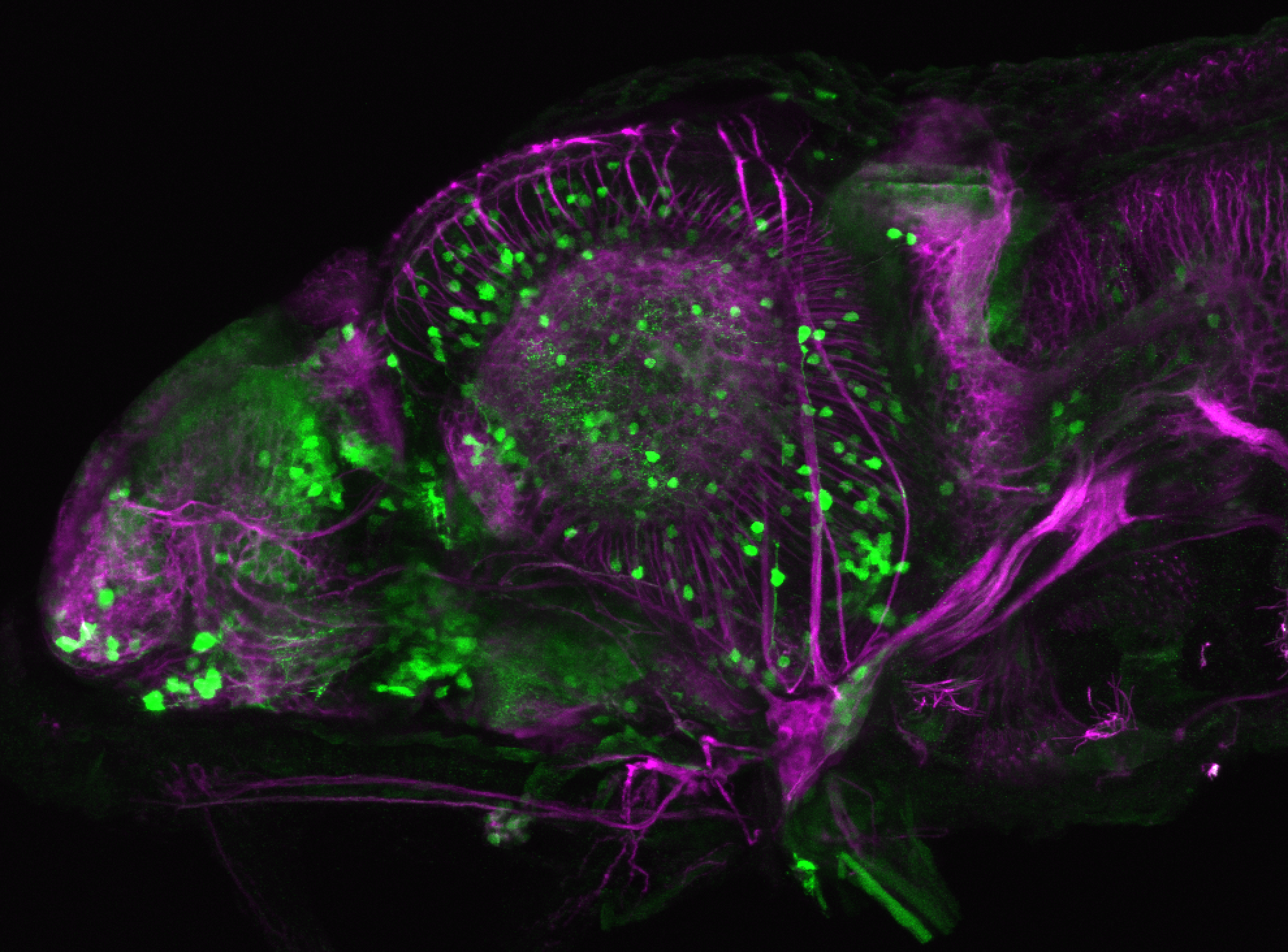
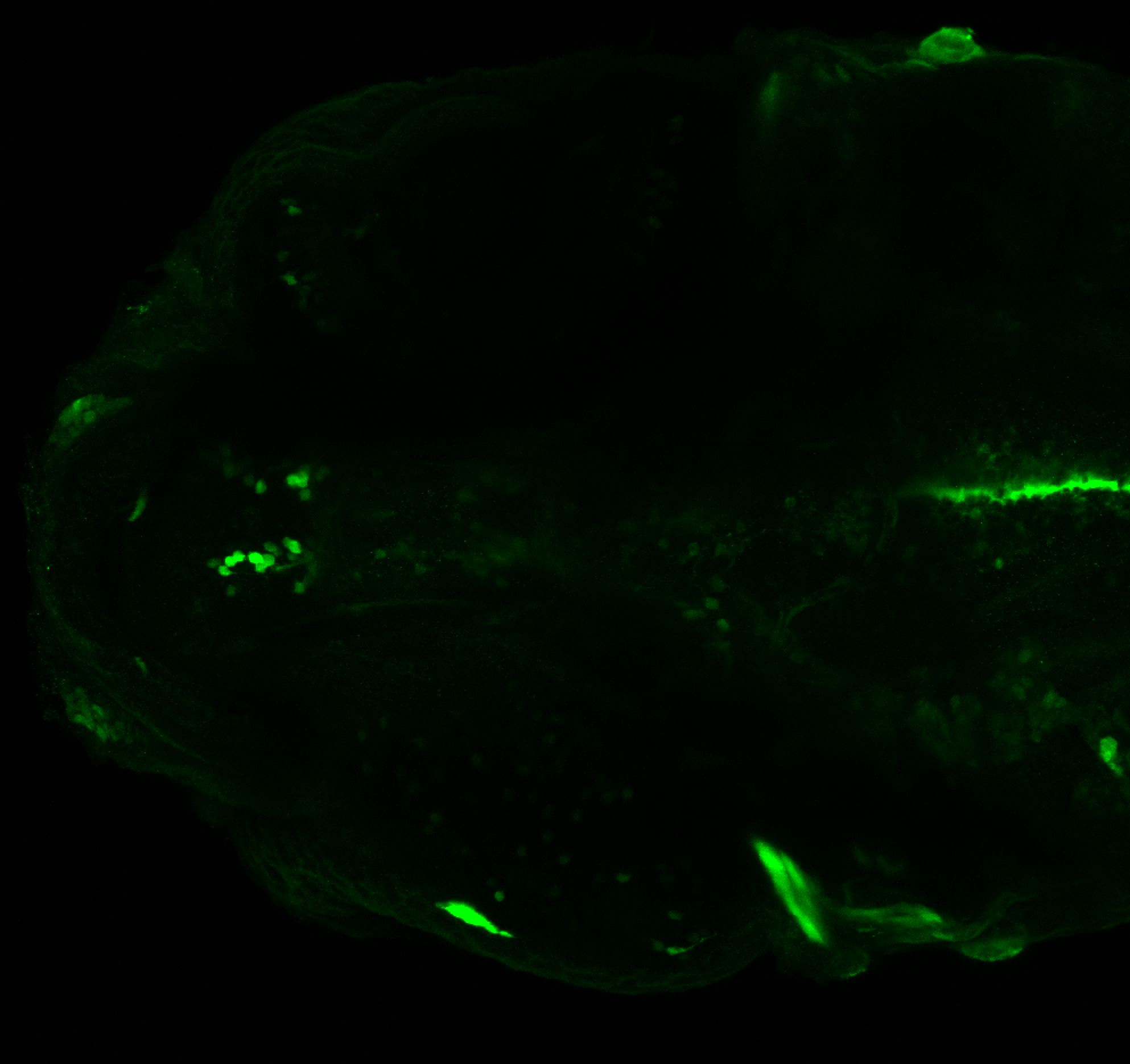
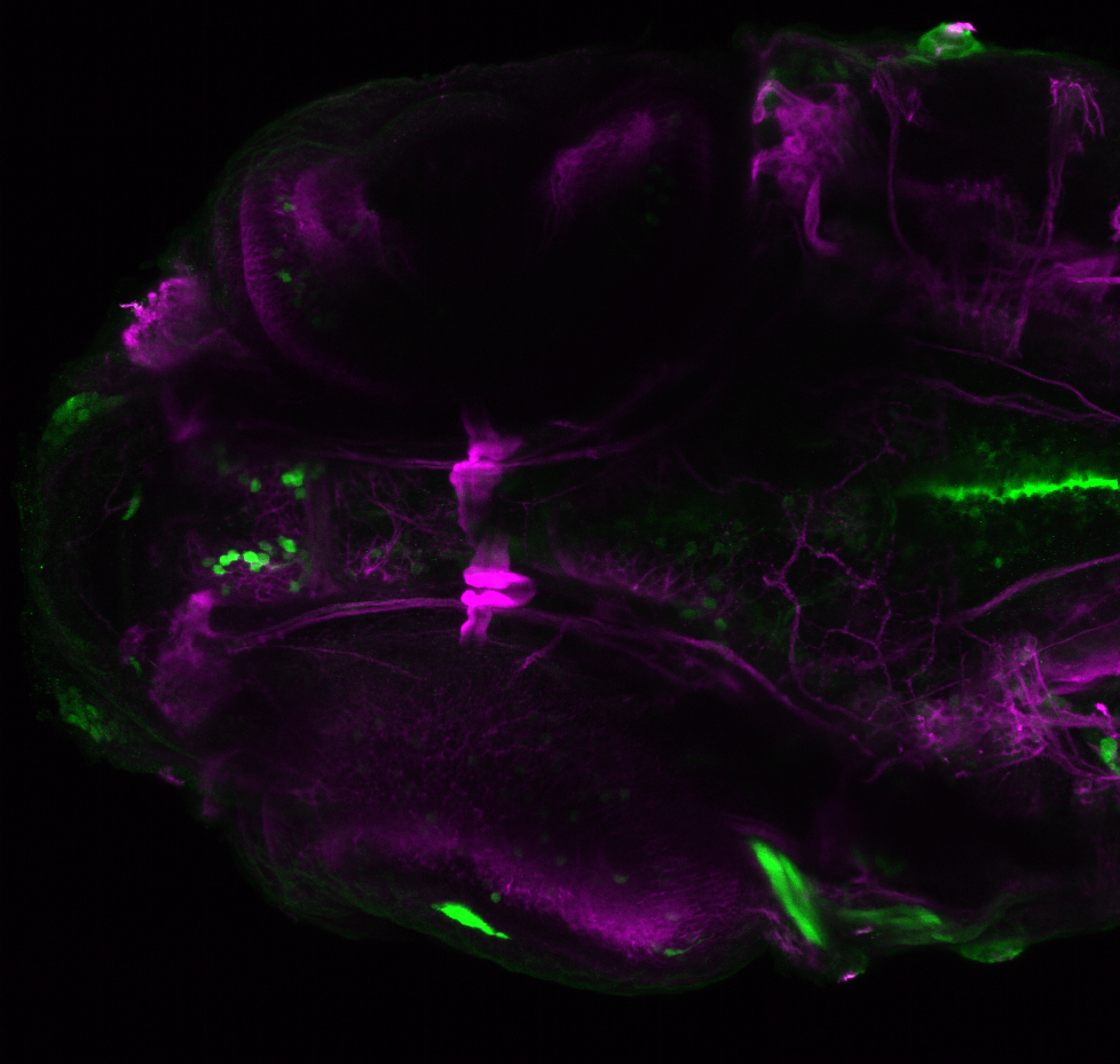
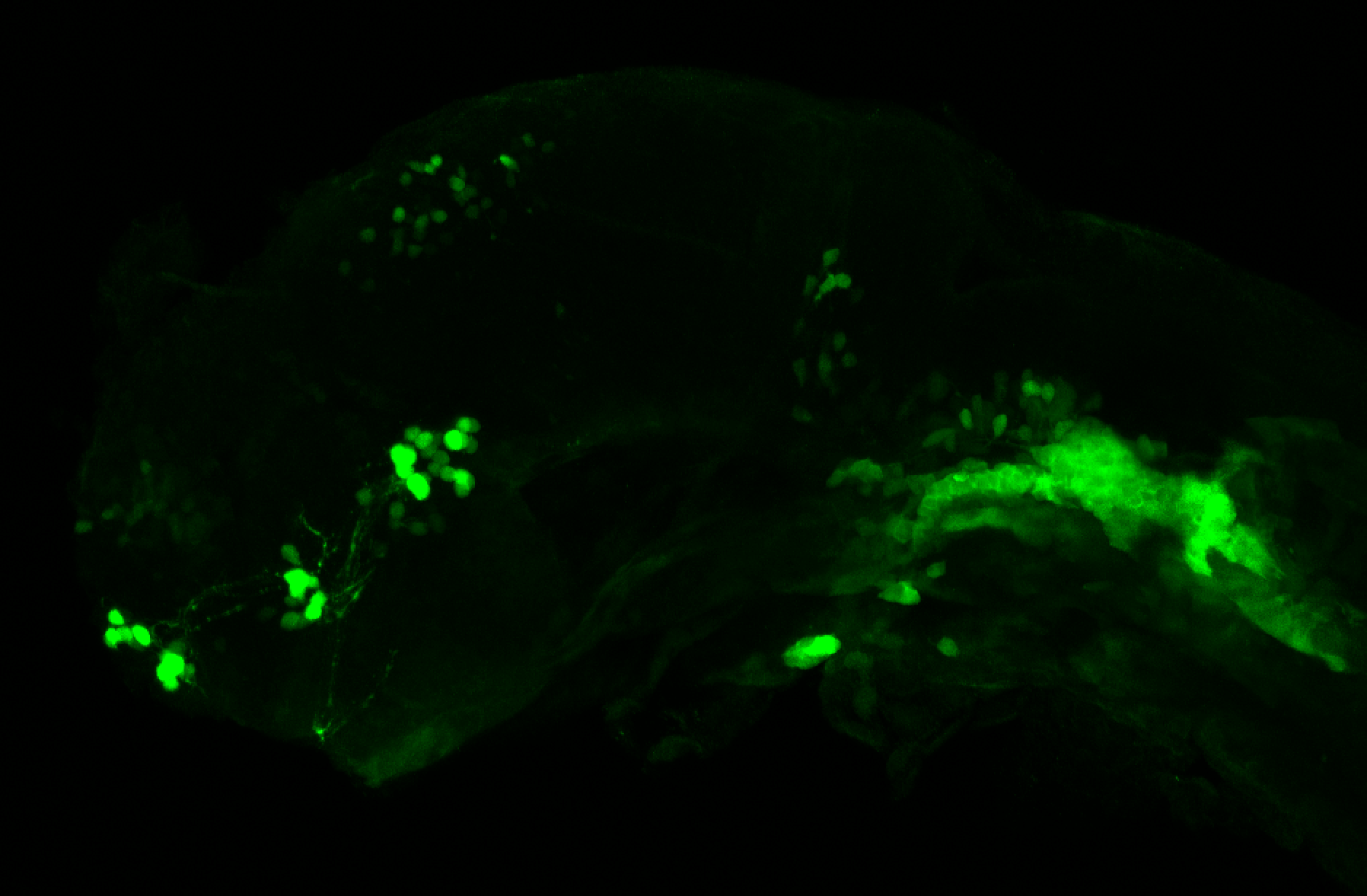
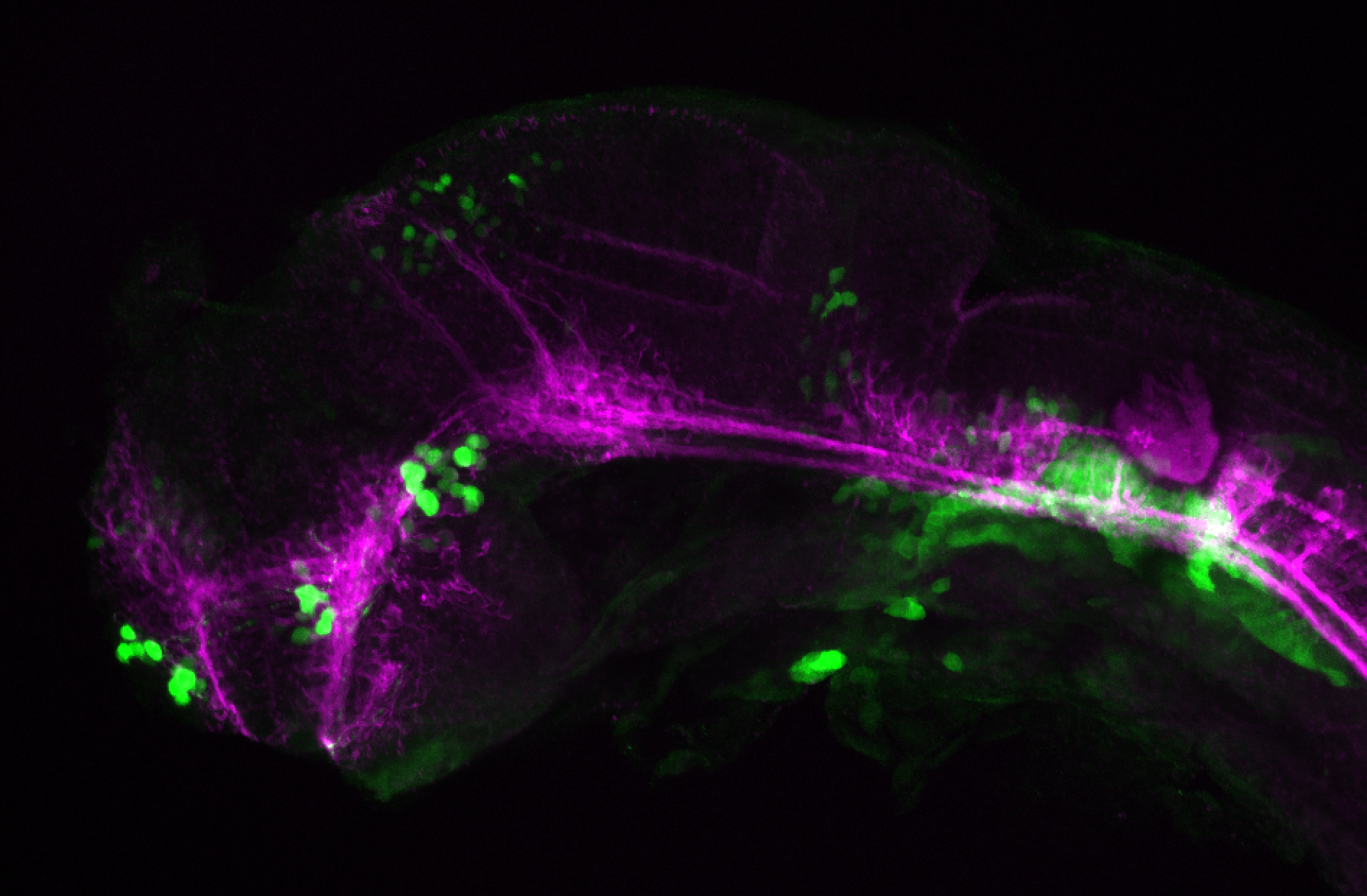
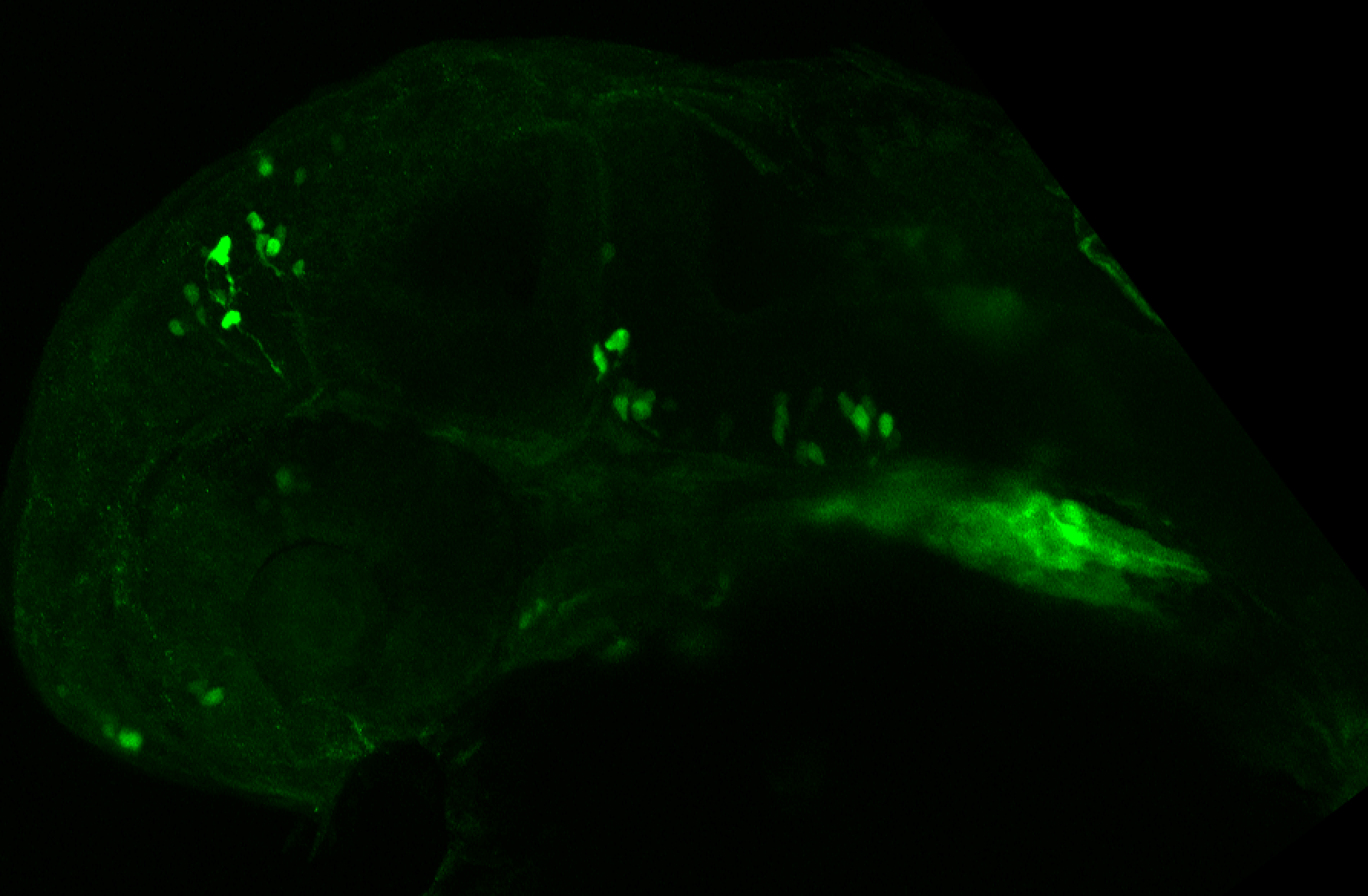
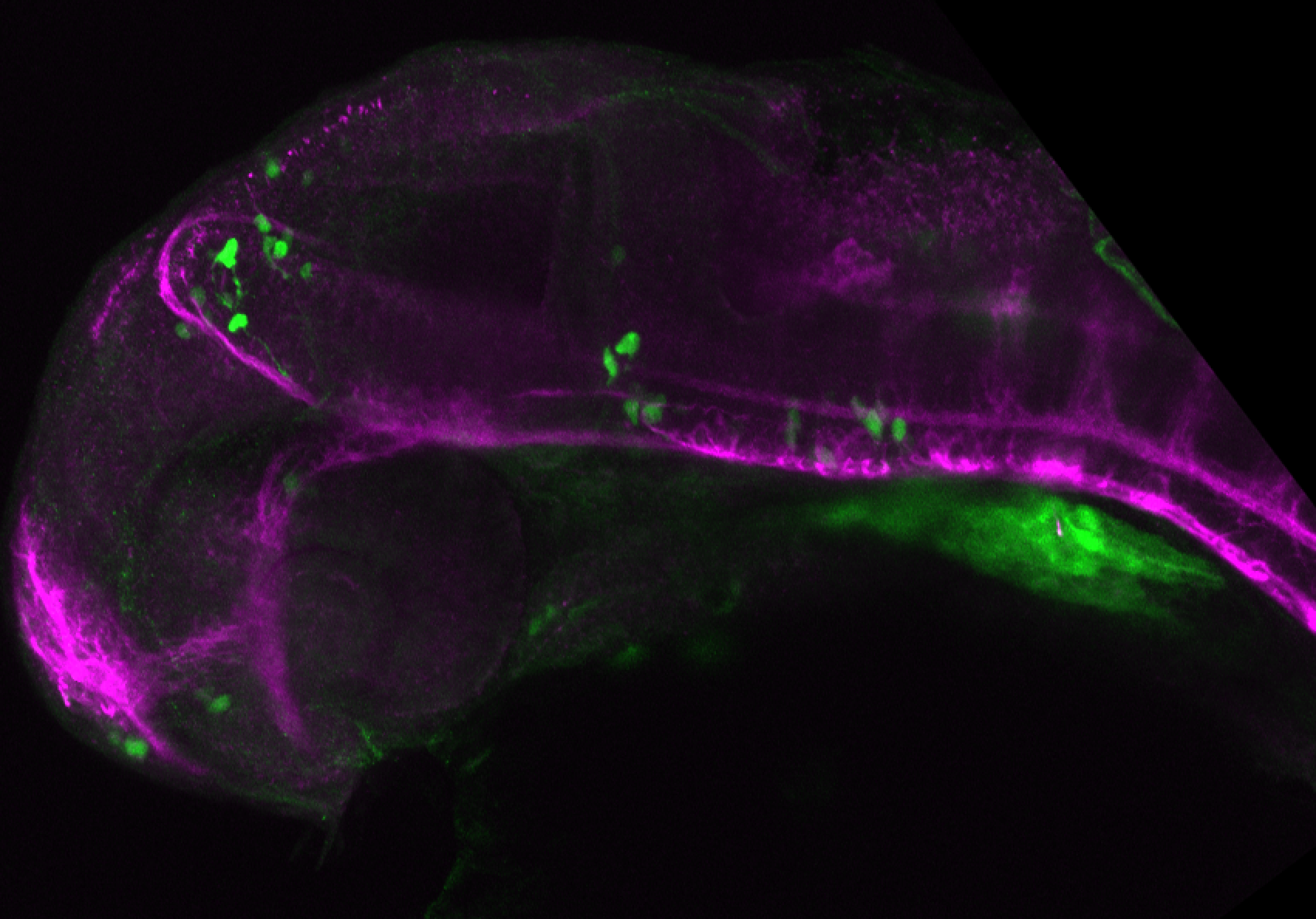
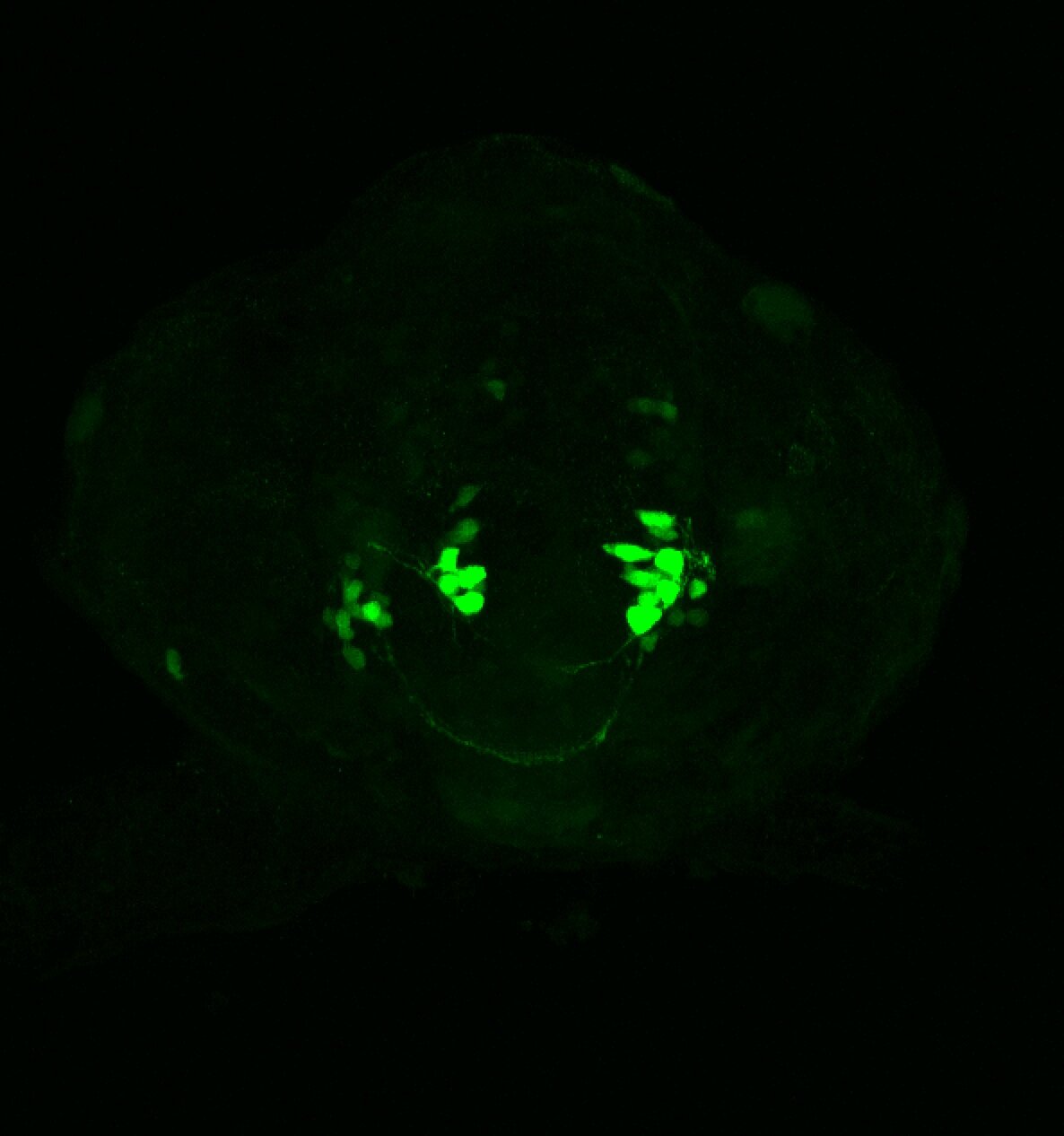
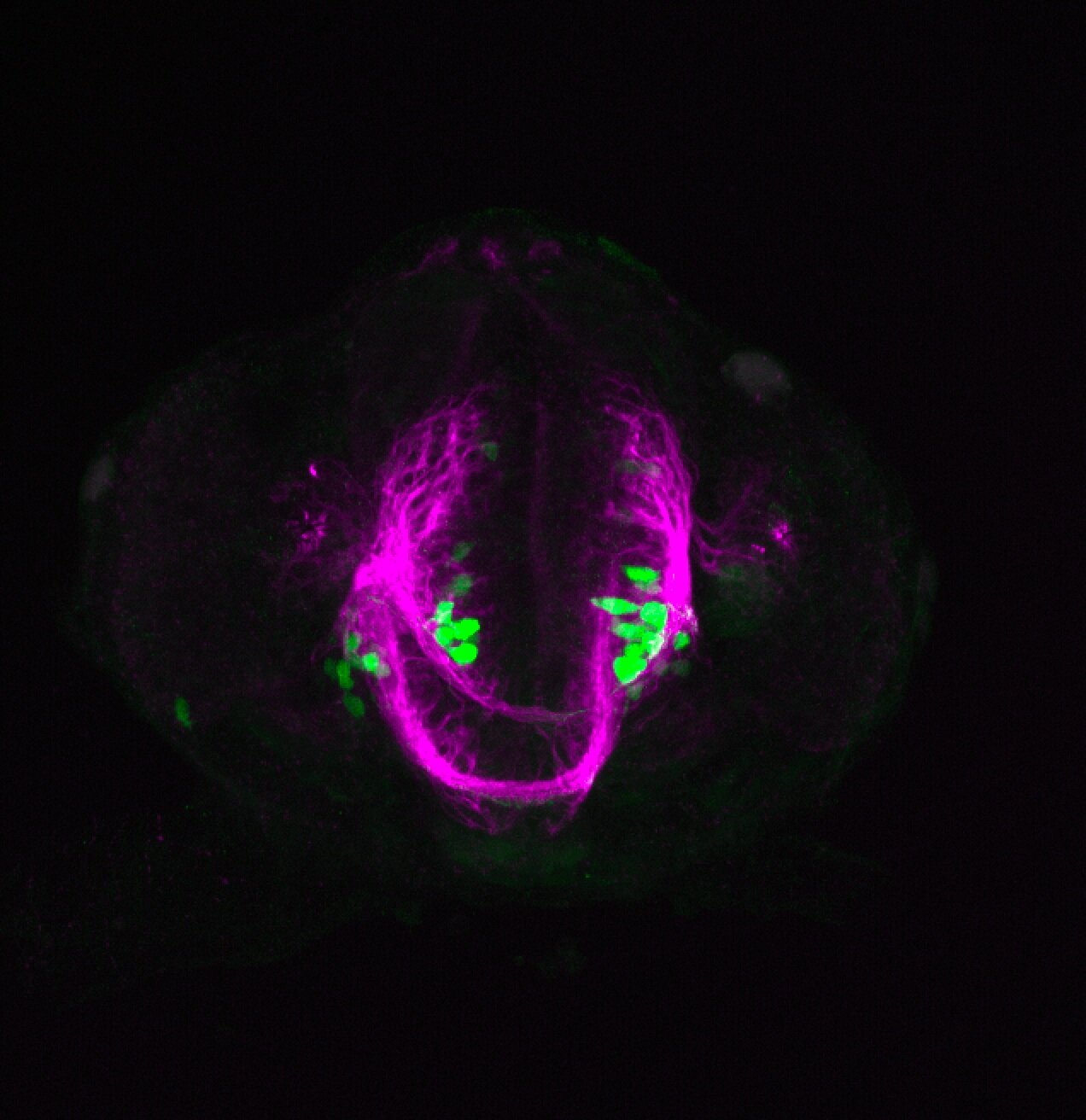
Enhancer trap line from Vladimir Korzh lab that has EGFP expression in the epithalamus, thalamic eminence, hypothalamic lobes, cerebellum, medulla oblongata.
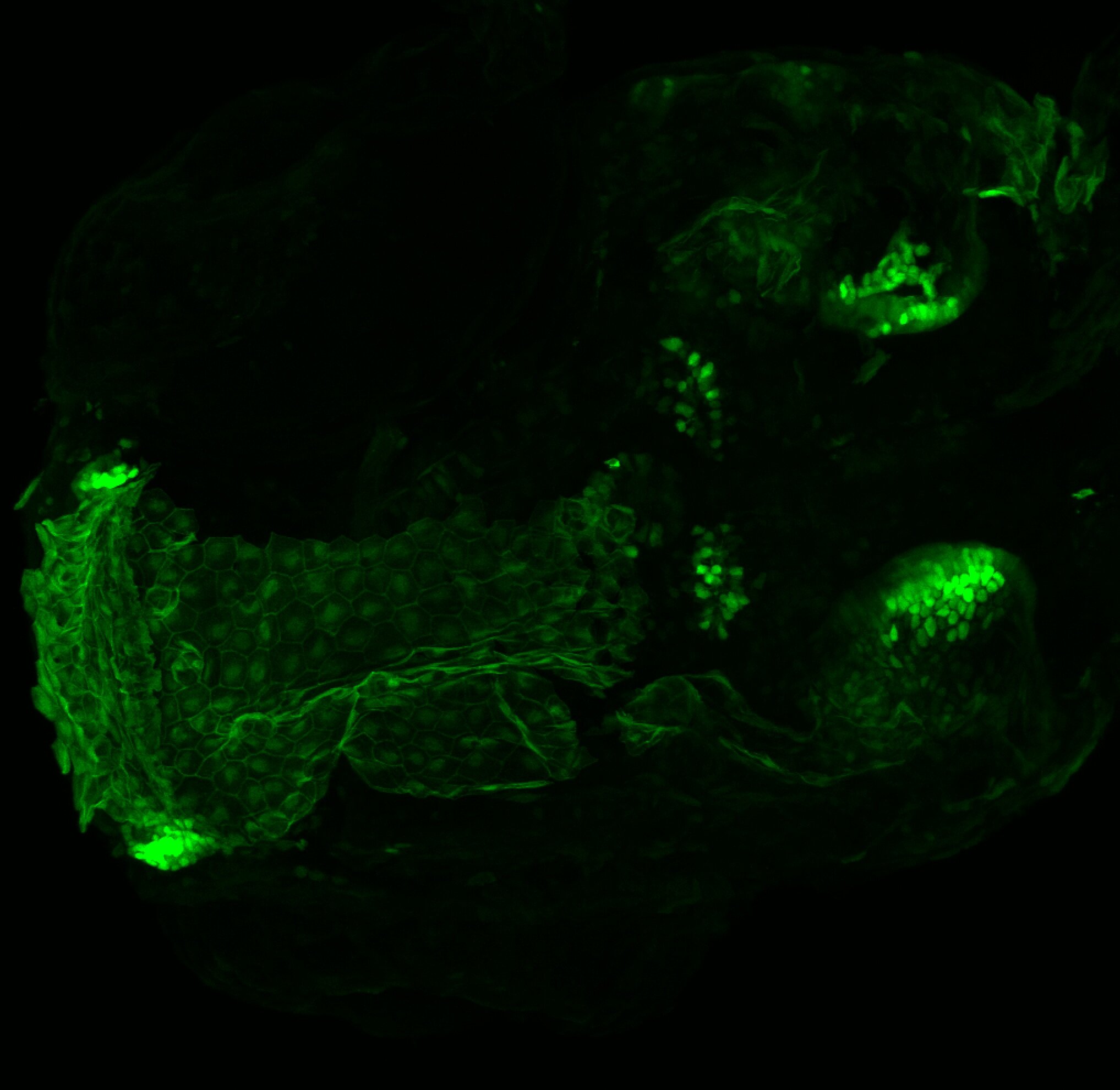
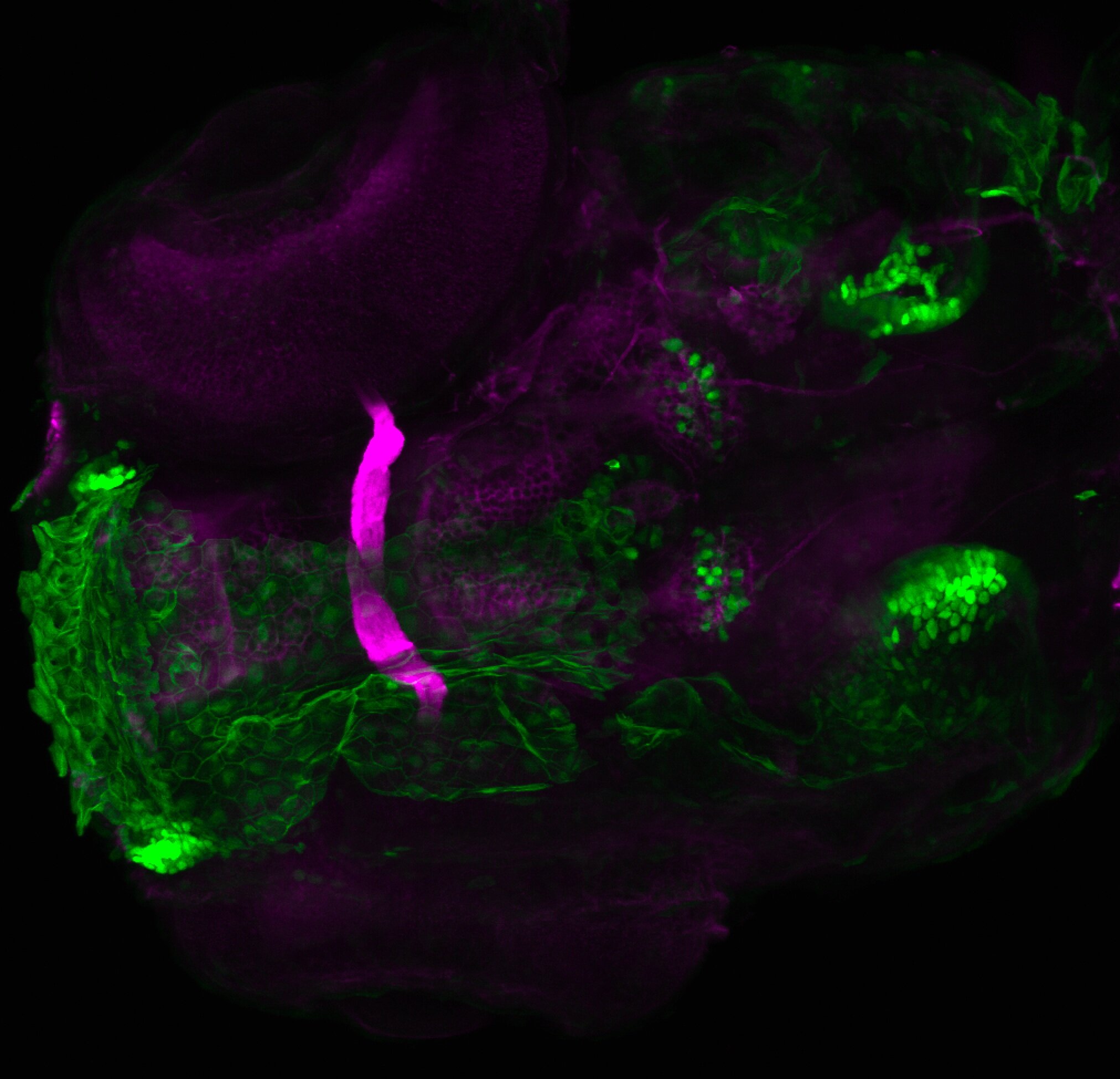
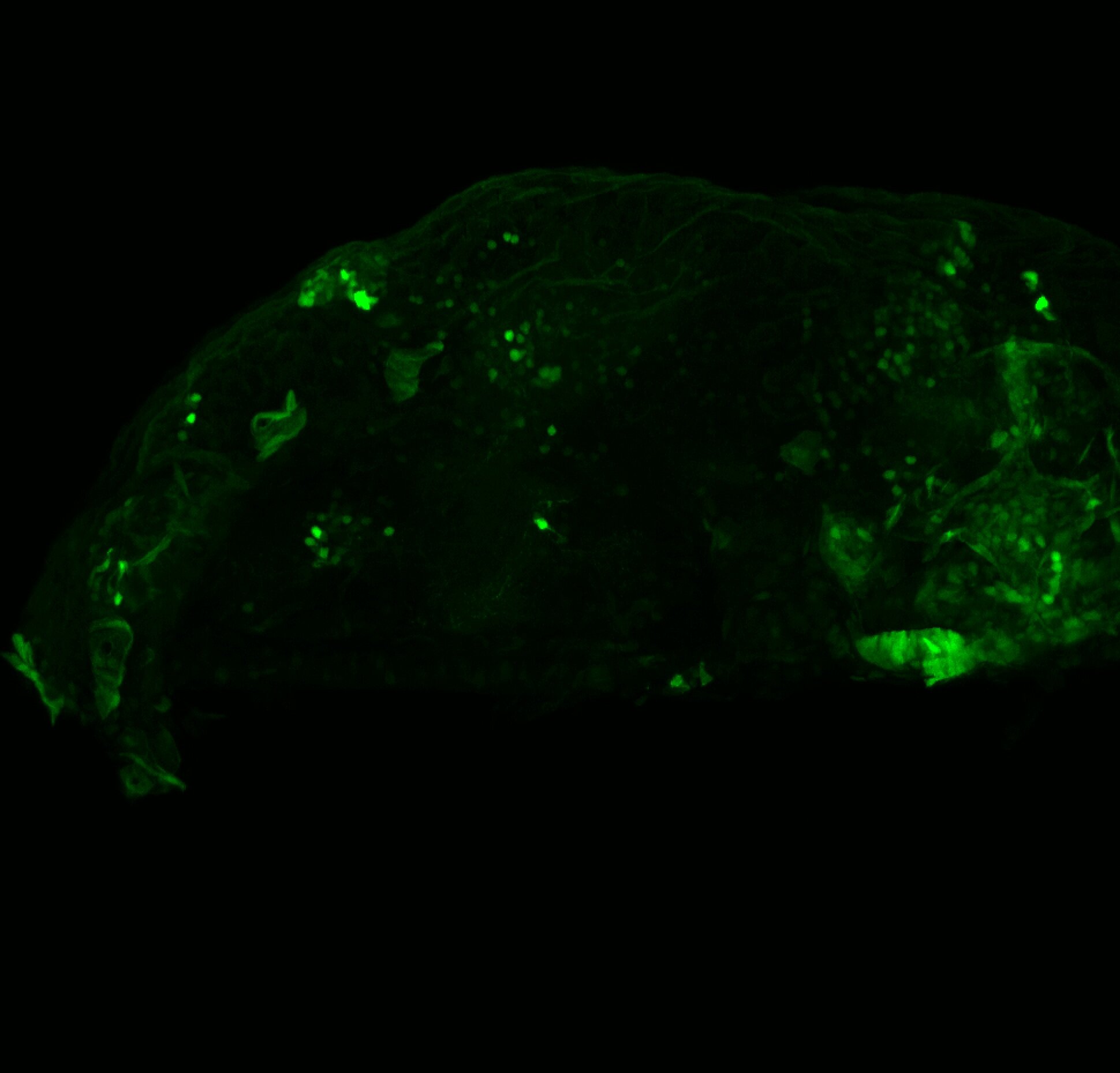
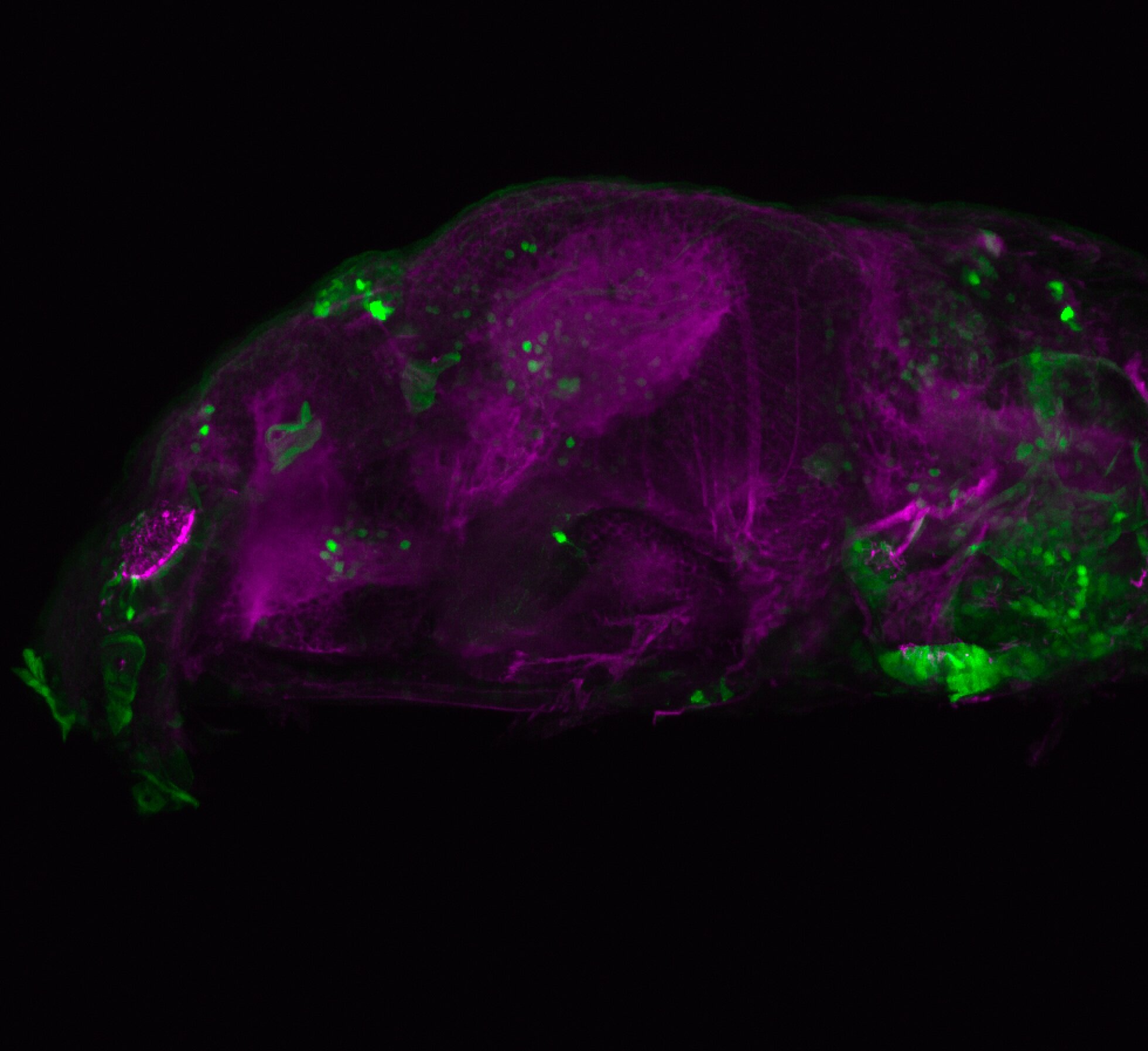
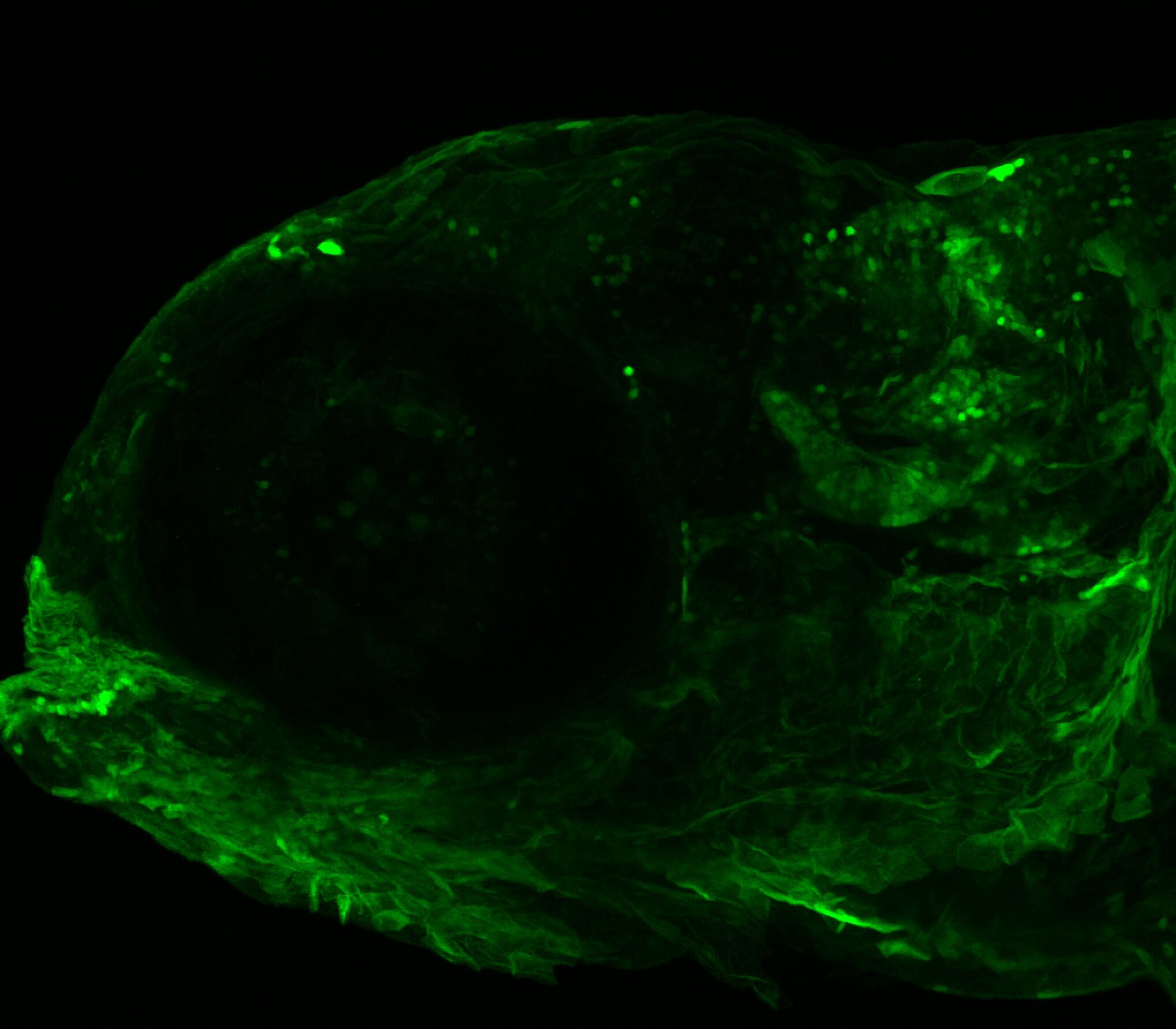
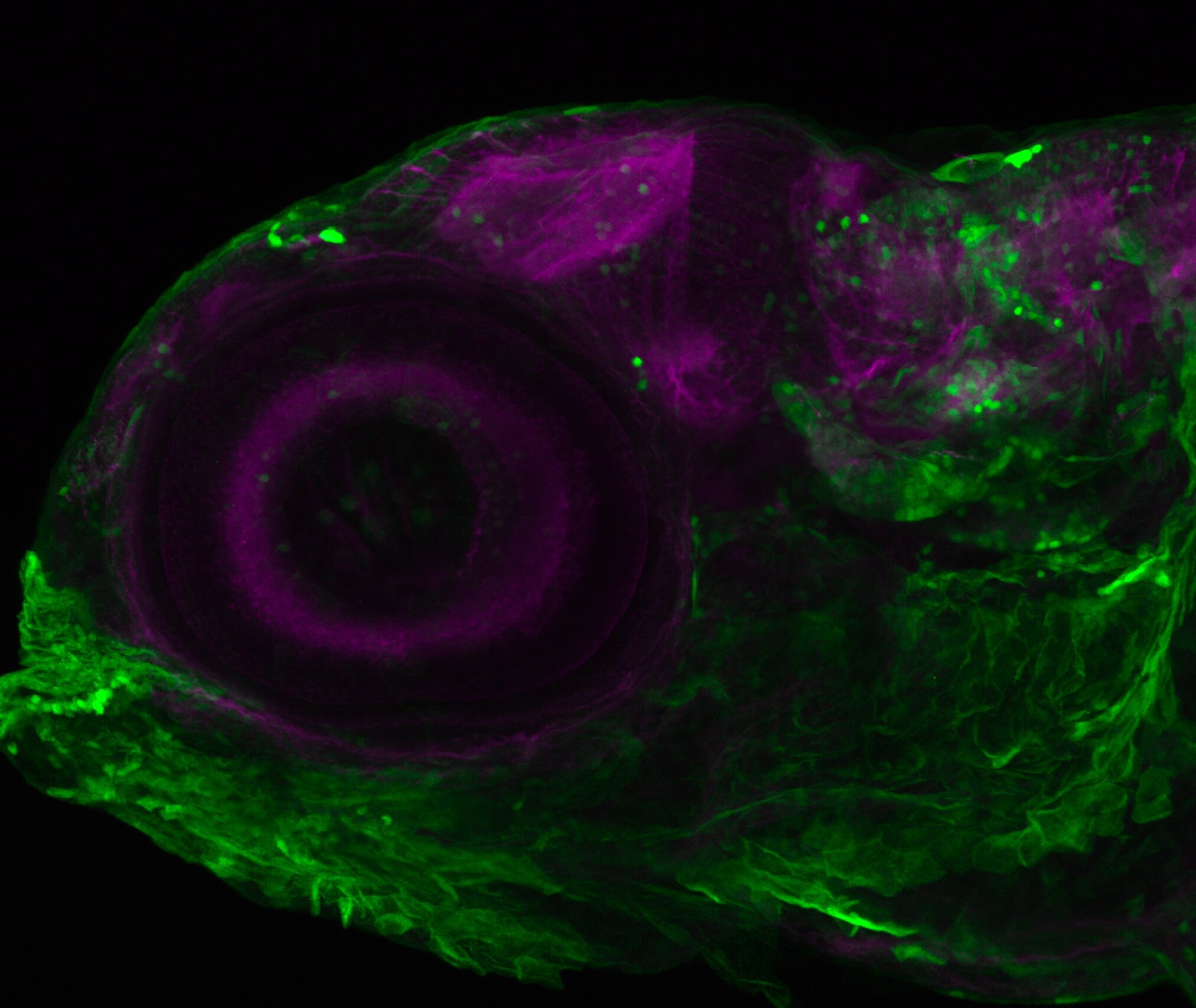
Expressed in:
epithalamus, thalamic eminence, hypothalamus, cerebellum, medulla oblongata
Key Publications
Parinov, S., Kondrichin, I., Korzh, V., and Emelyanov, A. (2004)
Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo.
Developmental dynamics : an official publication of the American Association of Anatomists. 231(2):449-459.