Glycine is an inhibitory neurotransmitter. Glycinergic transmission is found particularly in the retina, brainstem and spinal cord. Following activation of the glycinergic receptor, negatively charged chloride anions enter the postsynaptic neuron leading to an inhibitory postsynaptic potential. Glycine also acts as a co-agonist for glutamatergic signalling through NMDA receptors.
Antibodies that label glycinergic neurons
Transgenic lines that label glycinergic neurons
Tg(glyt2:GFP) or Tg(slc6a5:GFP) can also be viewed on ZBB at ZBB>Aliases>Neurotransmitters>Glycinergic
Probes that label glycinergic neurons
Key Publications
Jusuf, P.R., Almeida, A.D., Randlett, O., Joubin, K., Poggi, L., and Harris, W.A. (2011)
Origin and determination of inhibitory cell lineages in the vertebrate retina.
The Journal of neuroscience : the official journal of the Society for Neuroscience. 31(7):2549-2562.
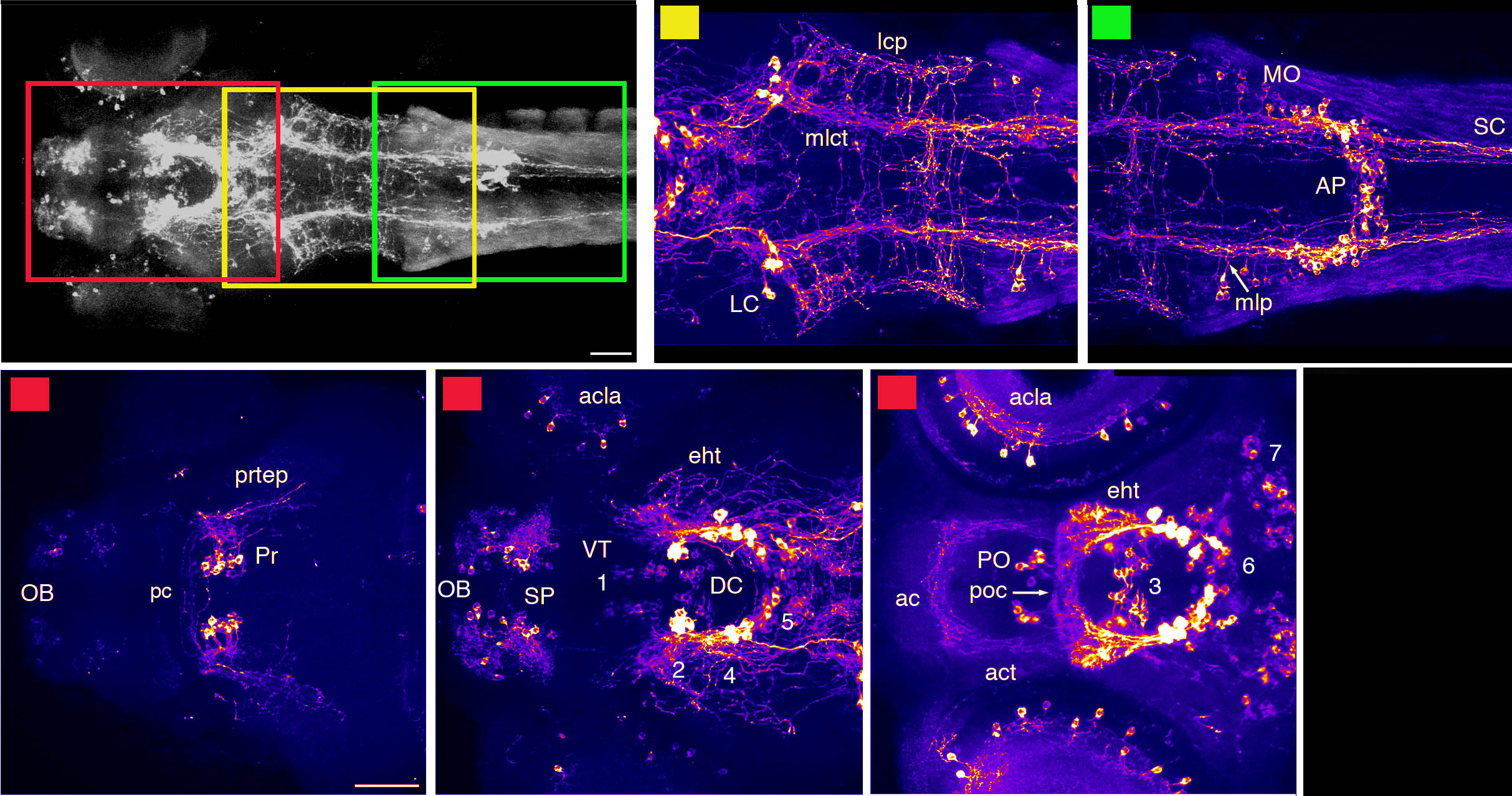
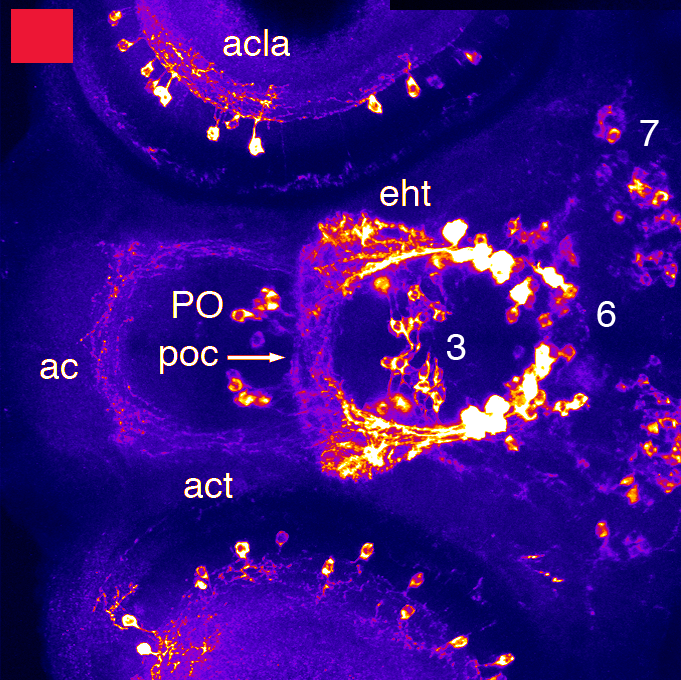
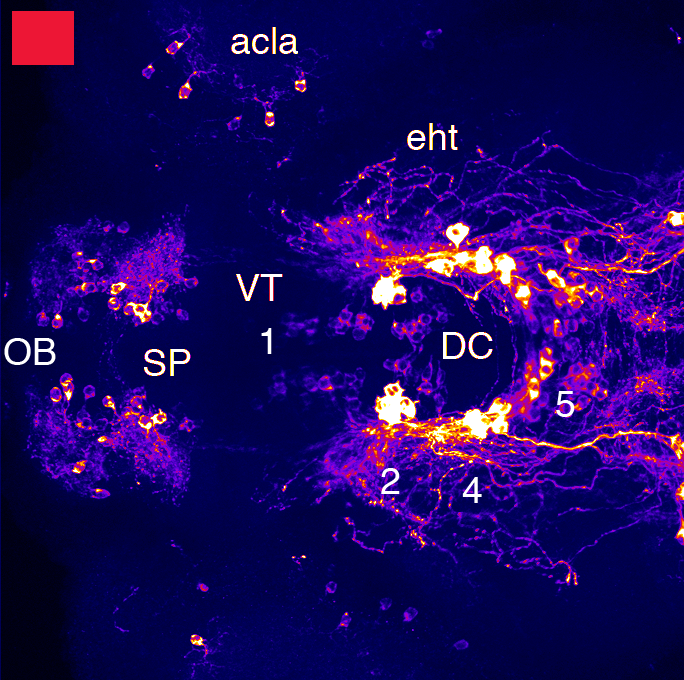
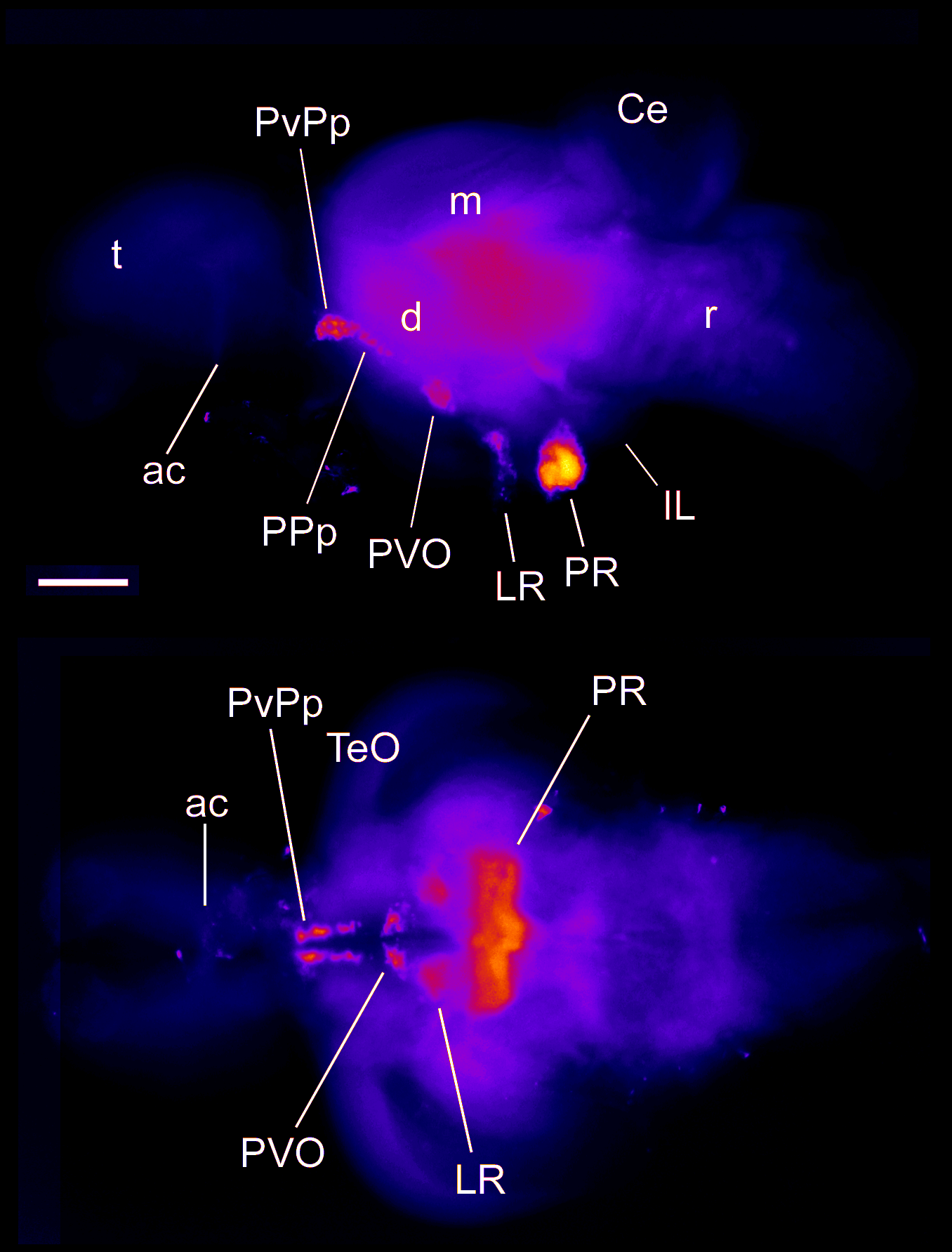
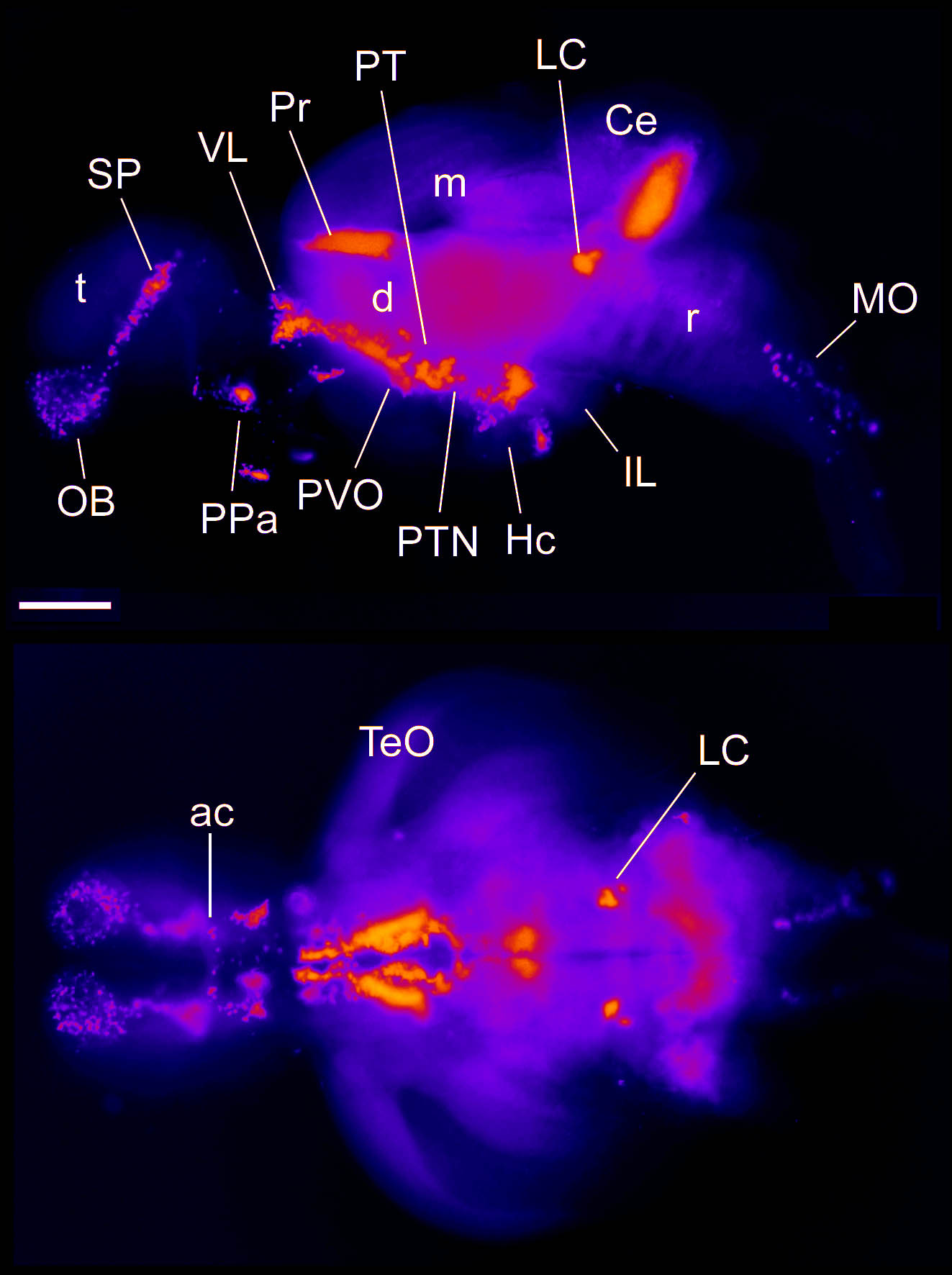
Barreiro-Iglesias, A., Mysiak, K.S., Adrio, F., Rodicio, M.C., Becker, C.G., Becker, T., and Anadón, R. (2013) Distribution of glycinergic neurons in the brain of glycine transporter-2 Tg(glyt2:gfp) transgenic adult zebrafish: Relation with brain-spinal descending systems.
The Journal of comparative neurology. 521(2):389-425.