This publication summary relates to the paper:
Cachd1 interacts with Wnt receptors and regulates neuronal asymmetry in the zebrafish brain (Powell et al., 2024)
Download our new paper in Science from UCL Discovery.
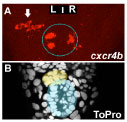
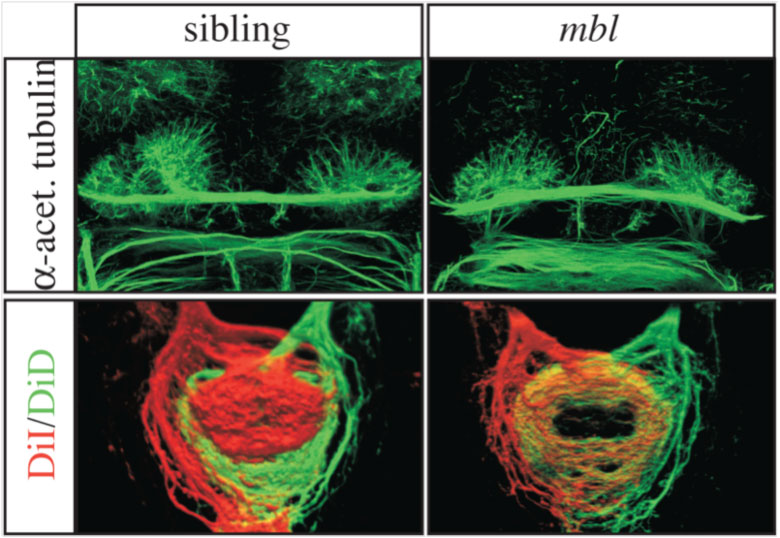
The human brain is divided into left and right hemispheres that superficially look like mirror copies of each other. However, there are differences between the sides that ultimately affect the types of neural circuits formed, the function of those circuits, and our overall behaviour. Many of these lateralised differences are also found in other animals and appear to have been conserved in evolution. One example of such conservation is the habenulae: two small brain nuclei, one present on either side, that act as a relay station to integrate sensory information and then pass a signal on to other circuits that control some forms of motor behaviour. We study the habenulae in zebrafish because there are easy to observe left-right asymmetries in the translucent embryo, such as size and shape, gene expression and circuit inputs and outputs (see figure below, A-C: compare wildtype panels on the left with mutant panels on the right). How those differences arise, and what affect they have on behaviour, is still not very well understood. In the Wilson lab, we look at how asymmetries are established in the brain during embryonic development by exploring what genes are involved in the process, what effect those genes have on the structure of the habenulae, and finally, how the zebrafish behave.
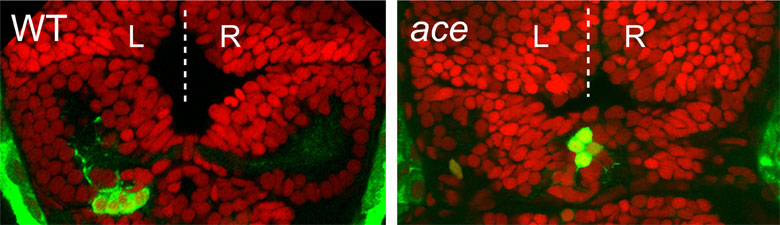
We have recently discovered that a gene called cachd1 plays a very important early role in establishing the difference between the left and right habenulae: when it is mutated, the right habenula develops into an almost mirror copy of the left habenula (see figure below, A and B). We have learnt that cachd1 does this, at least in part, by controlling the time at which the habenula neurons are born. Normally, more neurons are born early on the left-hand side than the right, and this affects the character of the mature habenulae (see figure below, D, left panel). When cachd1 does not function, more neurons are born early on both sides (see figure below, D, right panel), leading to mature habenula that look alike, so-called “double left” habenulae. This in turn affects the type of sensory circuits that can connect to the habenula, and how the habenulae connect to downstream circuits (see figure below, C).
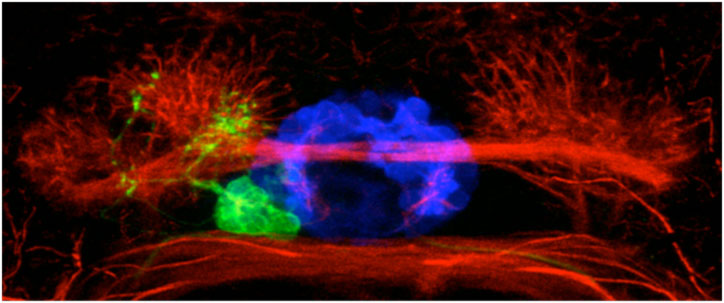
We believe that cachd1 is able to control the timing of neurons by modulating a key developmental signalling pathway called the Wnt pathway. Wnt is a secreted protein that simultaneously binds to two receptor proteins (Frizzled and LRP6) on the surface of cells. This interaction sets off a chain reaction of events inside the cell, passing information to the nucleus allowing the cell to react to its environment. Wnt signalling is involved in lots of different developmental processes in the embryo, typically (but not exclusively) to do with controlling the proliferation of cells. Similar to the Wnt protein, Cachd1 protein is able to physically bind to the Frizzled and LRP6 receptors (see figure below, E), potentially competing with the signal to alter the output of the pathway; in this case, the time at which a habenula neuron is born. Consistent with this, mutation of lrp6 also causes “double left” habenulae to develop (see figure below, F).
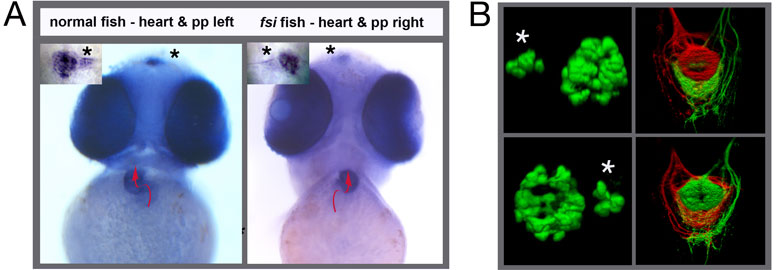
Interestingly, cachd1 isn’t limited to either side of the brain in the embryo, so how does it control asymmetry? We believe that the function of cachd1 is suppressed by a signal from the parapineal – a small population of neurons that migrates to the left-hand side and contacts the left habenula (see [Ingrid’s paper summary]). When the parapineal is ablated, or cannot function properly due to mutation, the left habenula develops as a mirror copy of the right habenula, so-called “double right” habenulae (see figure below, G, left panel). However, when the parapineal is ablated in embryos lacking cachd1 function, “double left” habenulae develop (see figure below, G, right panel). This means that the parapineal must inhibit cachd1 in order for the left habenula to form in the embryo.
This is an important piece of the puzzle, but lots remain to be discovered. We are particularly interested in learning about the consequences of the cachd1 mutation on behaviour, as well as developing a better understanding of how the Cachd1 protein affects Wnt signalling and what the identity of the antagonising parapineal signal is. Furthermore, our discovery of the function of cachd1 will help us uncover more genes that play roles in determining left-right asymmetry.
Loss of function of cachd1 disrupts asymmetry of the habenulae. Mutation of cachd1 (right panels A-D, G; compare to wildtype, left panels) affects asymmetry of gene expression (A) and neuron composition (B; neurons in red, Cachd1 protein in cyan) of the habenulae (dashed box in A), as well as connections of the habenula neurons to other brain regions (C; left side neurons in magenta, right side neurons in green). Neurons are born early in cachd1 mutant embryos on both sides (D), with an increased proportion of left-character neurons on the right (magenta). When neurons are born in the habenulae depends (in part) on Wnt signalling through Frizzled (FZD) and Lrp6 co-receptors. Cachd1 protein can bind to these simultaneously, shown in the crystal structure of the complex formed by the three proteins (E). Mutation of lrp6 also affects asymmetry in the habenula (F), consistent with its interaction with Cachd1. When the parapineal is ablated in a wildtype embryo, both left and right habenula express a gene characteristic of the right habenula (G, left panel, green; pineal gland shown in white); this suggests that a signal from the parapineal is needed to break asymmetry. When it is ablated in the cachd1 mutant however, “double left” habenulae form; this suggests that cachd1 is needed to form the right habenulae, and must be suppressed by the parapineal signal on the left.