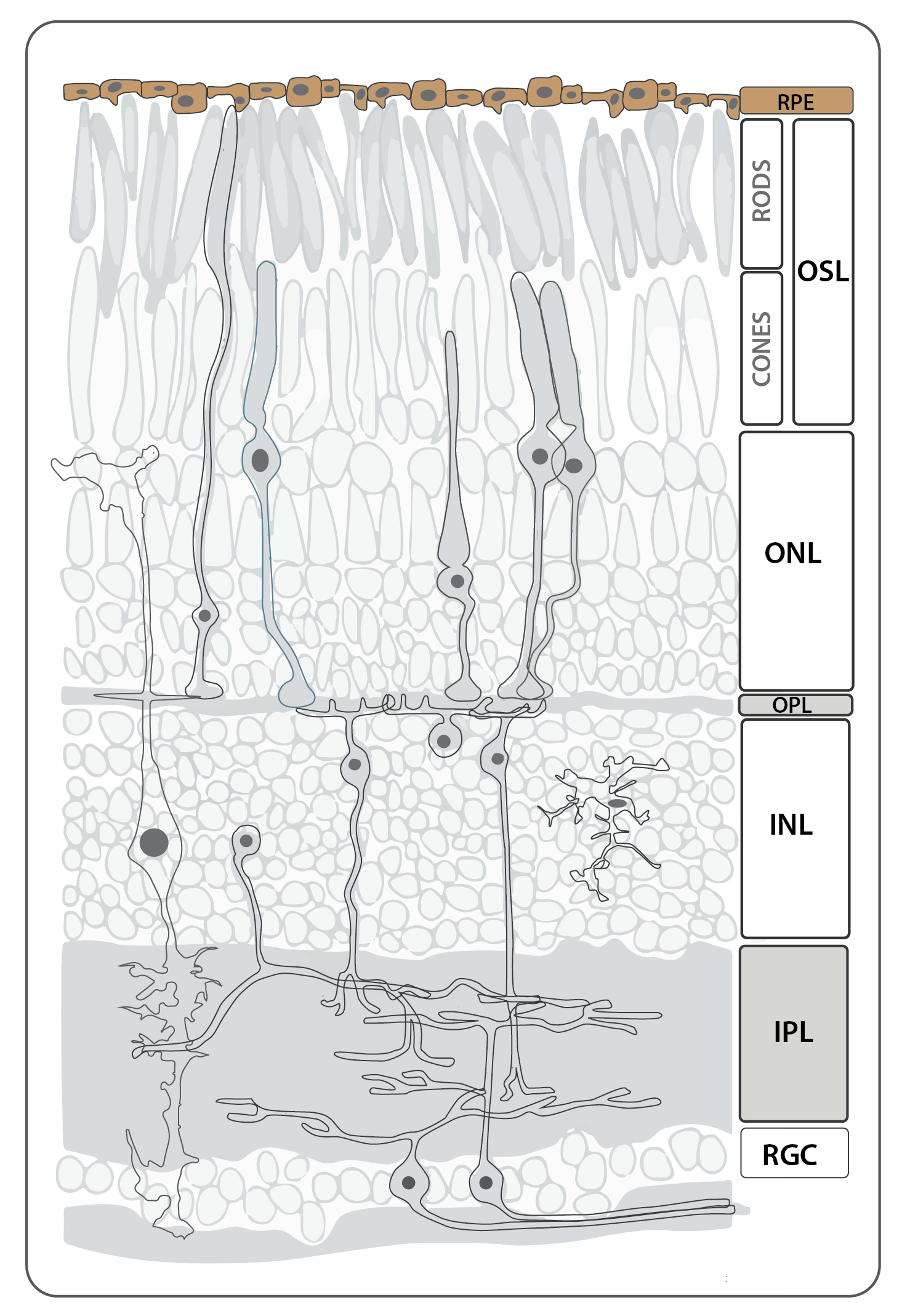
The retinal pigmented epithelium (RPE), like the neural retina, is derived from the neural ectoderm, and through complex morphogenetic movementsis during development it becomes tightly apposed to the photoreceptor cell layer of the neural retina. This tissue is required for the function and survival of photoreceptor cells; numerous mutations affecting vacuole transport in the RPE result in non-cell autonomous photoreceptor cell degeneration.
The RPE is a typical epithelial monolayer of tightly packed cuboidal cells forming part of the blood brain barrier between the vascular choroid and the neural retina. The cells contain large numbers of melanosomes packed with melanin granules. The apical microvilli of the RPE interdigitate with the photoreceptor outer segments, and the basal surface rests on Bruch’s membrane, the basement membrane between the RPE and capillaries of the choroid.